Opioid overuse is one of the major public health crises of the 21st century in the Western world. Since 2000, nearly 600,000 people have died from an opioid overdose in Canada and the United States, giving rise to the opioid epidemic. By 2029, the death toll could reach 1–2 million.
Opioids, a class of pain-relief drugs that can be natural, synthetic, or semi-synthetic, mitigate the sensation of pain. They include the legal prescription drugs oxycodone (OxyContin), hydrocodone (Vicodin), codeine, and morphine, as well as illegal drug heroin, all of which can be highly addictive, potentially leading to opioid use disorder and overdose.
This catastrophic loss of life not only impacts families and communities but also strains the healthcare system and the economy, amplifying the urgency of the situation.
Opioids: Pain relief with a high risk
On a biochemical level, the therapeutic and euphoric effects of opioids result from their binding to mu-opioid receptors, which are found in the central and peripheral nervous systems as well as the gastrointestinal tract. The stimulation of these receptors causes opioid-induced respiratory depression, a state in which breathing slows and becomes shallower. In the event of an overdose, breathing may stop altogether unless treatment is given in time.
In particular, the number of overdoses owing to the synthetic opioid fentanyl has dramatically risen in recent years. Fentanyl is about 50 to 100 times more potent than morphine, meaning its lethal dose is comparatively small.
Although the drug is FDA approved and prescribed for severe pain, it’s also illegally manufactured and mixed into other street drugs, like cocaine and heroin, making it especially insidious. In New York City alone, fentanyl was responsible for 80% of overdose deaths in 2021.
Given this worsening crisis, public health experts are calling for the implementation of radical measures to quell the wave of overdose deaths, and researchers are investigating safer alternatives to traditional opioids that effectively treat pain with lower addiction potential.
One way is to make opioid reversal drugs readily available without a prescription. The drug naloxone, which prevents the activation of mu-opioid receptors and is the preferred treatment for opioid overdose, is both accessible and easily administered.
In fact, the naloxone-containing nasal spray Narcan has recently been FDA approved and is the first opioid overdose reversal drug to be sold over the counter at pharmacies, convenience stores, and supermarkets across the United States.
The widespread availability of Narcan and its generics is projected to reduce the prevalence of overdose fatalities in the immediate term, as social spaces where overdoses tend to occur, such as bars and restaurants, can now easily keep the drug on hand, allowing bystanders to quickly intervene at the first signs of an overdose.
A human-based model for studying opioid overdose and recovery
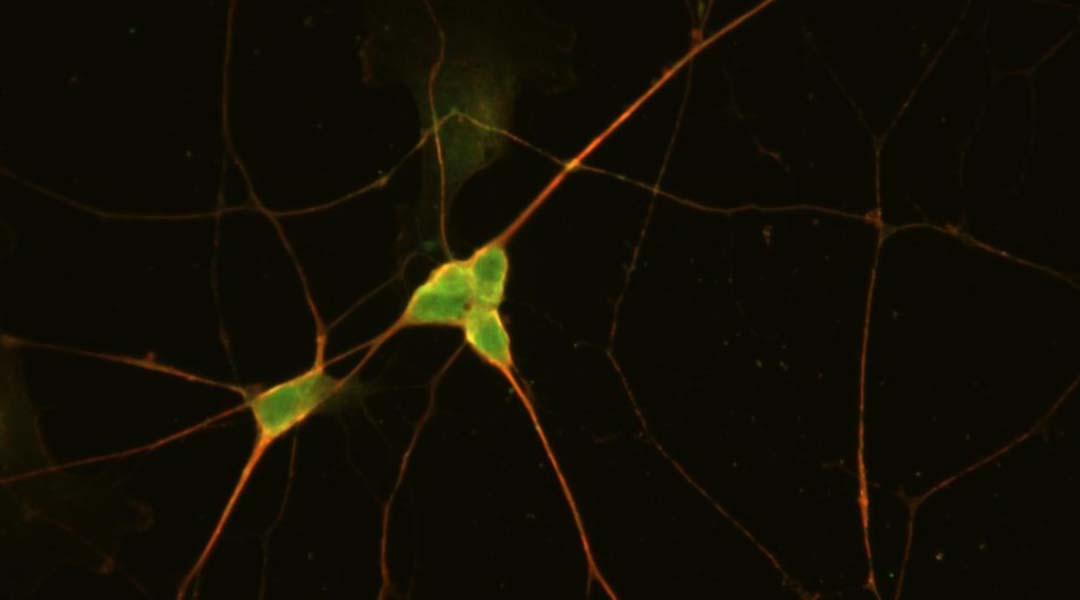
Understanding opioid-induced respiratory depression is key to developing more effective treatments for opioid overdose, and a team of Florida-based researchers have recently taken steps toward this goal. They developed the first human-based model of a neural network located in the brainstem called the preBötzinger complex (preBötC), which is essential for breathing and survival in mammals.
Opioids induce respiratory depression by suppressing the function of the preBötC, which is the main cause of opioid-related deaths, so investigating its role is crucial. Their human-based model offers distinct advantages over previously developed animal models.
“It offers the flexibility of investigating preBötC neural network properties and its interactions with other respiratory centers, as well as its regulation by different modulators, such as CO2 or O2,” James Hickman, a chemistry professor at the University of Central Florida who helped develop the model, explained. The previously developed animal models don’t offer this level of flexibility.
The preBötC is very small, which makes differentiating preBötC neurons challenging. In their model, human stem cells known as iPSCs were differentiated into neurons with preBötC-like features.
“Our in vitro iPSC-preBötC model provides a system that reproduces the response of these neurons to opioids, investigates the regulation of the preBötC network activity under physiological and pathological conditions, and evaluates the effects of therapeutics,” Hickman told us.
Development and application of the iPSC-preBötC model
To develop the opioid overdose model, Hickman and colleagues evaluated the dose dependency of methadone, codeine, fentanyl, and a synthetic opioid peptide, DAMGO, on the inhibition of iPSC-preBötC-like neurons.
To do this, they tested the opioids using “whole-cell patch clamp electrophysiological recording”, which involved delivering a puff of a certain dose of the opioid into the vicinity of the neuron and recording the neuronal firing. The graphs generated from these recordings revealed how the neuronal activity changes with opioid dose.
The researchers then evaluated the effect of naloxone on the iPSC-preBötC-like neurons, simulating the reversal of opioid-induced respiratory depression during overdose. This involved dosing the neurons with the opioids until the neuronal activity was completely inhibited and monitoring the recovery of neuronal activity after introducing different naloxone dosages.
Based on their results, the researchers determined the most effective naloxone dosage for each opioid, which is imperative because naloxone overdose can inhibit the recovery of neural activity from opioid inhibition. Their results were on par with previous findings for in vivo rat models, validating their model.
Providing a deeper understanding
Hickman also mentioned that their model has been adapted into a microelectrode array system for higher throughput, allowing the efficacy and toxicity of various opioids and therapeutics to be investigated under a range of physiological and pathological conditions, such as inflammation and stress.
This system could eventually be combined with other organ mimics to better understand organ–organ interactions during opioid overdose and recovery. Drug–drug interactions and patient risk factors that can increase the risk of opioid-induced respiratory depression — for example, sleep apnea — can also be investigated using this model as a basis, helping to establish more specific guidelines for primary care clinicians.
But gaining a better understanding the effects of opioid overdose on the body is only one piece of the puzzle — solving the opioid epidemic will require efforts from all sides. The social stigma surrounding opioid overuse disorder first needs to be removed: it must be recognized as a serious medical condition.
Keeping clinicians up to date about the current guidelines for prescribing opioids must also be integrated with interventions that consider the mental, physical, material, and social needs of the individual. All of these measures will save lives.
Reference: James J. Hickman, et al., Human IPSC-Derived PreBötC-Like Neurons and Development of an Opiate Overdose and Recovery Model, Advanced Biology (2023). DOI: 10.1002/adbi.202300276
Feature image: Immunofluorescent image of iPSC-preBötC neurons stained with mu-opioid receptors (red) and NK1R antibodies. Credit: Xiufang Guo
This article was updated September 29, 2023 to correct the quote attributions from Guo to Hickman.