With more than 400,000 new cases diagnosed worldwide each year, endometrial cancer is the sixth most common cancer in women and the most frequent type of cancer in the female reproductive tract.
It is thought to begin in a layer of cells that form the lining of the uterus, called the endometrium, and arises from an accumulation of mutations that confer cancerous features, namely non-stop cell growth, increased survival, and the ability to invade and colonize other tissues.
The combination of mutations that create cancer cells are called driver mutations, which are “those that confer to the cell an advantage over its counterparts, for instance making it resistant to cell death,” said Xavier Dolcet, principal investigator at the Biomedical Research Institute at the University of Lleida in Catalonia, Spain.
Now, in a study published in Advanced Science, the research group led by Dolcet has discovered that mutations in two genes — PTEN and P53 — are responsible for the development endometrial cancer. The researchers used the CRISPR/Cas9 system, also famously known as genetic molecular scissors, which are able to remove, edit, or even add genes to living systems.
They used them here to delete PTEN and P53 in mouse models they developed. “This means a more accurate representation of the disease’s initiation and progression,” explained Raúl Navaridas, former Ph.D. student at Dolcet’s lab and first author of the publication. “This level of control is lacking in many previous models.”
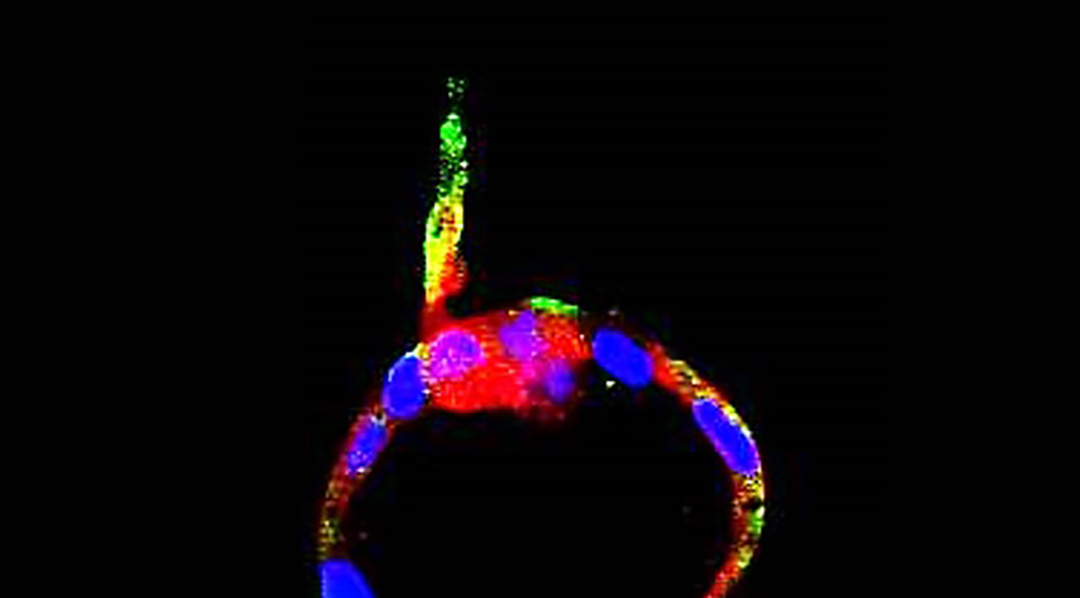
It is the first genetic model of endometrial cancer with such a high level of precision, as in most previous models, all cells contained the mutation, not just those found in the endometrium.
“Our mouse models recapitulate quite well human endometrial cancer,” added Dolcet. “It simulates both the histopathology — the characteristics of the tumor that can be observed using a microscope — and the development and aggressiveness of human uterine carcinosarcomas — the rarest and most aggressive form of endometrial cancer currently lacking models of study.”
PTEN mutations must occur first
When studying the genetic origins of cancer, hundreds, thousands, or even millions of mutations can be found in cancerous cells. However, understanding which of these mutations cause disease is a hard task.
Clinicians may make some estimations due to the frequency, position, or length of a mutation, but it is not easy to distinguish them.
“The data obtained from genomic profiling of endometrial cancer left us large lists of genes that appear to be mutated in cancer,” said Dolcet. Therefore, an essential question in cancer biology, which Dolcet and his research group were trying to answer, is how do we know the contribution of each mutation to the cancer?
Some driver mutations have a bigger impact than others, and while some would only alter a gene’s function, others might completely shut down its activity. There is the added difficulty that driver mutations do not appear all at once. Cancer cells mutate progressively and in a similar way, the accumulation of mutations might make cells more susceptible to mutation.
Dolcet explained that since each mutation contributes to a different tumor feature, driver mutations need to occur in a sort of chronological order, which affects disease progression and aggressiveness. “For instance, each subtype of endometrial cancer may have different [driver mutations] associated with it,” added Navaridas.
The study revealed that while cutting out both PTEN and P53 genes was enough to cause aggressive forms of endometrial cancer, the outcome was very different when the mutations were generated individually. Deleting only PTEN generated benign tumors whereas the deletion of P53 was not enough to produce any tumors at all.
“Human cancer is the result of the accumulation of mutations, and a single driver mutation may not be in itself sufficient to cause cancer,” said Dolcet. However, Dolcet confessed these are surprising results since most aggressive endometrial cancers contain P53 mutations.
“PTEN mutations contribute to increasing proliferation — cell division — and therefore are an early event in the development of endometrial cancer,” he continied. “In contrast, P53 mutations may result in genomic instability, a process that leads to the accumulation of a large number of mutations.”
Driver mutations must therefore appear first in PTEN for the disease to initiate while P53 mutations are needed in advanced stages of endometrial cancer when cancer cells need to acquire different properties to invade other parts of the body. “The combination of mutations is the one responsible for the progression of the disease,” Dolcet concluded.
A better model of endometrial cancer
In many genetic models of cancer, genes are mutated in every single cell. “[When] all the cells of the tissue have the [cancer] mutation,” explained Dolcet, “that does not leave healthy cells in the tissue.” However, that does not represent the real disease context. “In the body, cancer cells are surrounded by other non-mutant cells,” he explained.
An advantage of using the CRISPR/Cas-9 system is that any genetic “cut” can be done in select cells. “Inducing mutations only in some cells [of the endometrium] and leaving other cells of the tissue completely normal, provides a better approximation to the genetic diversity of human endometrial cancer,” said Dolcet.
“Sometimes, experimental treatments that are effective in treating mice cancer, do not work that well in humans, unfortunately,” he continued.
With a more realistic recreation of endometrial cancer in mice, Dolcet expects the results obtained from this animals to be more relevant to the human disease. “This mouse model could become a good platform to test the efficacy of new anticancer drugs,” he concluded.
Reference: Xavier Dolcet, et al., In Vivo Intra-Uterine Delivery of TAT-Fused Cre Recombinase and CRISPR/Cas9 Editing System in Mice Unveil Histopathology of Pten/p53-Deficient Endometrial Cancers, Advanced Science (2023). DOI: 10.1002/advs.202303134
Feature image credit: Xavier Dolcet et al.