The past few decades have witnessed a dramatic advance in organic syntheses from the viewpoint of environmental science and technology. Click chemistry was one of these advances, featuring mild reaction conditions, high efficiency, tolerance of a wide range of functional groups, and easy purification. As represented by the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC), the so-called “click” reactions have actively been employed in materials science.
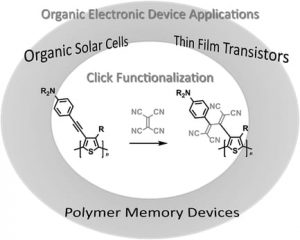 In a review article published in Macromolecular Chemistry and Physics, Professor Tsuyoshi Michinobu (Tokyo Institute of Technology) highlights a novel click reaction inspired by semiconducting polymer doping techniques. Cyano-based acceptor molecules, such as tetracyanoethylene (TCNE) and 7,7,8,8-tetracyanoquinodimethane (TCNQ), are known as conventional p-type dopants of semiconducting polymers to increase conductivity. However, these molecules also undergo [2+2] cycloaddition-retroelectrocyclization with electron-rich alkynes in a click chemistry fashion, forming novel tetracyanated acceptor units.
In a review article published in Macromolecular Chemistry and Physics, Professor Tsuyoshi Michinobu (Tokyo Institute of Technology) highlights a novel click reaction inspired by semiconducting polymer doping techniques. Cyano-based acceptor molecules, such as tetracyanoethylene (TCNE) and 7,7,8,8-tetracyanoquinodimethane (TCNQ), are known as conventional p-type dopants of semiconducting polymers to increase conductivity. However, these molecules also undergo [2+2] cycloaddition-retroelectrocyclization with electron-rich alkynes in a click chemistry fashion, forming novel tetracyanated acceptor units.
The author highlights the chemical and electronic properties of the tetracyanated acceptors in organic electronic devices. For example, it is shown that the small molecular-weight tetracyanated products are effective organic photosensitizers in dye-sensitized solar cells (DSSCs). In contrast to the conventional organic photosensitizers requiring a carboxylic acid anchoring group to adsorb onto the TiO2 surface, the tetracyanted units can serve as both a physical anchoring group and an electronic acceptor. This will simplify the molecular design of organic photosensitizers.
The author further highlights the applications in polymer thin film devices. The precursor aromatic polymers containing electron-rich alkynes are carefully designed by activating the alkynes using aromatic amines and organometallic subunits. Click functionalization of these polymers results in a decrease in their energy levels, and the extents of the decrease are controlled by the amount and species of the added acceptor molecules. Interestingly, the energy levels are correlated with the device performances of thin film transistors and polymer solar cells. Moreover, excellent volatile and non-volatile polymer memory performances are also presented.
In a final note, Prof. Michinobu suggests future research directions. By utilizing the strong electron-accepting character, the development of compatibilizers in polymer solar cells as well as artificial photosynthesis systems seems to be promising.