Antibodies are a vital component of today’s clinical applications. For instance, many new and effective treatments that have recently been developed against cancer rely completely on the use of antibodies. These powerful molecules are also routinely used to detect and diagnose diseases, as antibodies have the ability to capture specific proteins, that are indicative of a certain disease. Furthermore, antibodies are indispensable for modern research, as they are one of the most powerful tools available for biological and clinical studies.
 The major bottleneck for the continuously increasing high demand for antibodies is the purification process. Conventional materials used to purify antibodies are expensive and
The major bottleneck for the continuously increasing high demand for antibodies is the purification process. Conventional materials used to purify antibodies are expensive and
have a very limited antibody binding capacity, which restricts the throughput. Also, the sensitivity of diagnostic kits for the detection of diseases is confined by low antibody binding capacities of the applied surfaces. Elevated performance of instruments and electronic devices is frequently attained through miniaturization of the involved components, which increases the number of functional units in a given volume. Analogously, materials can be endowed with a higher antibody binding capacity if the number of binding sites for the molecules is magnified by increasing the surface area over volume ratio.
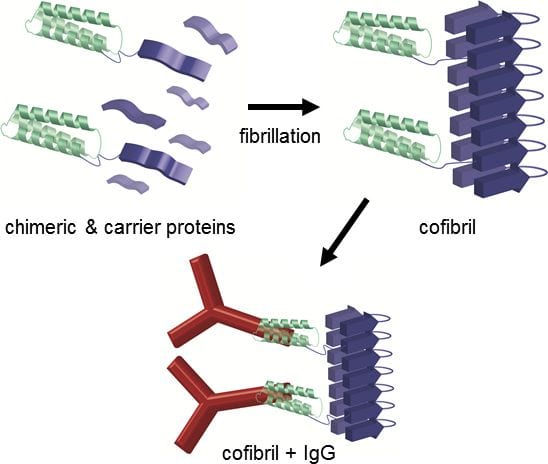
A recent article by Benjamin Schmuck et al. in the group of Torleif Härd (Uppsala, Sweden) in Biotechnoloy Journal describes an antibody binding material based on small proteins that are prone to spontaneously self-assemble into nanofibrils. These nanofibrils have a diameter of 5 nm, but at the same time possess a stiffness that has been compared to steel. For an improved antibody binding material that requires an enormous surface area over volume ratio, these properties are key features that make the protein nanofibrils an interesting scaffold for this kind of application. Through engineering of the DNA that encodes the fibrillating proteins, the authors equipped the fibrils with functional units that are able to bind antibodies. As a result, this new material has the capacity to bind almost 20-times more antibodies than any other commercially available material.
This study represents a first step towards the aim to provide more cost-effective means for antibody purification, which ultimately may lead to affordable monoclonal-antibody therapies. The fibrils can additionally be used to boost the sensitivity of medical diagnostic kits, which could lead to earlier and more reliable disease diagnosis.
















