Imagine a future where biosensors could be printed on demand at home and used without extensive medical experience for rapid diagnosis and care. Can Dincer is fully invested in making this future a reality. For Dincer though, his responsibility doesn’t stop with the publication of his work in academic journals, he is active on an astounding number of channels, escaping the academic bubble and finding new audiences for his research.
Dincer is currently a Junior Research Group Leader at the FIT Freiburg Center for Interactive Materials and Bioinspired Technologies and the Department of Microsystems Engineering (IMTEK), University of Freiburg. He and his team are pursuing the realization of personalized care in a simple manner through a range of projects from microfluidic diagnostics, to machine learning for processing the data from disposable biosensors, to breath analysis.
He has an impressive social media prowess, with channels on YouTube, Twitter and Instagram among many others. These platforms are used not only to disseminate his scientific knowledge, but also to talk with other scientists about how best to make their research reach further.
We sat down with Dincer to discuss his research endeavors, his social media footprint, and how he empowers future generations of scientists so that they can effectively harness their abilities.
What inspired you to become a scientist?
I think it was my curiosity. I was very curious as a child, always asking questions and I believe this led me to become a scientist.
After graduating from high school, I had two courses of study in mind that I was very interested in: biology and architecture. However, I then decided to study microsystems engineering, which at first glance actually doesn’t have that much in common with these two subjects.
During my studies, I have decided to work as a student assistant and there was a position for electrochemical sensors, which fascinated me. That was the beginning of a long story that led me to biosensors for different applications. Interestingly, now I build microstructures (like an architect) and combine them with (synthetic) biology (like a biologist) to determine diseases faster and more sensitively on site.
What do you love about your work?
I love to be free. I can wake up one day and say, “I would like to work on that topic” and start working on that with my group. I also love to be in touch with many people, including my own students, and help them on scientific and career issues. I try to answer all of the messages I get on social media or in emails, and I hope I can keep this up as the workload gets higher. I like to see young researchers who are inspired by science and to be able to work with them.
You are currently a group leader at the University of Freiburg. Can you tell us more about the work your group does with “disposable microsystems”?
We are developing easy-to-use and affordable sensors for on-site applications so that maybe one day, you can do it in your home with a wax printer using paper, for example. The necessary materials and equipment are not really available at home right now, but in future, this could change, for example by using more functional 3D printers.
Our current research can be divided into three areas: In one, we use microfluidic channels in combination with electrochemical biosensors to accelerate reactions and to achieve a multiplexed detection, getting some time advantage in order to make it faster and study different analytes, like one we will talk about later in this interview for the CRISPR-powered on-site COVID-19 management. Besides, we also try to measure hormones using our biosensors placed on a living animal, like a chicken, (semi)continuously. The idea here in the longer term is to create a device, similar to the wearable blood pressure systems, but capable of measuring biochemical signals over weeks.
The second area is wearable sensors, which usually focuses on sweat and interstitial fluid analysis using patches or tattoos. We are targeting another biofluid, exhaled breath, using our paper-based sensors. In my group, before the pandemic, we made a sensor-integrated adapter to a facemask that is capable of sensing chemicals (hydrogen peroxide as inflammation marker) in the breath, but you can also measure biomolecules like glucose from exhaled breath continuously and noninvasively. This is a very promising approach for analyzing many analytes in a non-invasive manner, however, with many challenges like sensitivity required.
We are also very interested in data science and how we can use it in combination with sensors. For example, wearable sensors provide us a huge amount of data and allow us to record the history, look up the current status and hopefully in the future make predictive diagnostics. We need data sciences to connect these all together. In a collaboration, we are studying the data of a clinical trial performed to demonstrate the impact of therapeutic drug monitoring of antibiotics. Using the basic data analysis, it was not possible to see this impact. We are now planning to publish an investigation of the same study from a data science perspective by using machine learning and already seeing the positive impact of therapeutic drug monitoring on patient recovery rates.
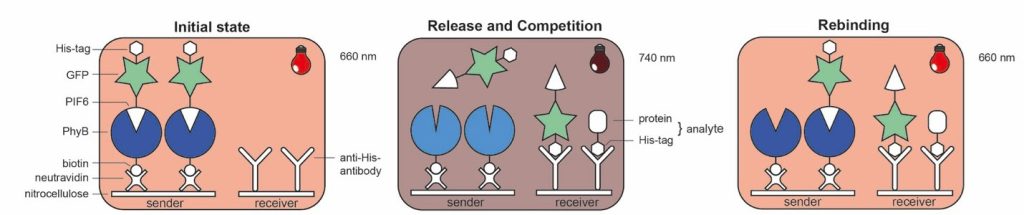
The third block is working with tools coming from synthetic biology, specifically optogenetic switches, which researchers use to control gene expression or cell signaling. We use one of them, the plant photoreceptor phytochrome B (PhyB) along with its interacting factor 6 (PIF6), which you can bind and release by simply changing the wavelength of light, in order to create an optically controllable assay, which we call “OptoAssay”. In our first approach (see figure), we tested a competitive assay where you do not need any microfluidics, you just change the wavelength and the competitor (here, his-tagged PIF6 fused with green fluorescent protein – GFP) is released and after the competition rebound again which eliminates the necessity of a washing step (see OptoAssay Working Principle).
Your lab also built a CRISPR-based COVID-19 test. What inspired this? How does the test work and how does it compare to existing COVID-19 tests?
The inspiration came from our work detecting microRNAs and we published our first paper involving this and CRISPR before the pandemic. CRISPR is a powerful tool to measure nucleic acids, even without any isothermal amplification. Once the CRISPR effector protein (in our work, Cas13a) recognizes the target, it gets activated and, due to its ability to cleave all non-target nucleic acids (in case of Cas13a, RNAs) in its surrounding, we introduce a so-called reporter nucleic acid (here, a reporter RNA) which can generate an electrochemical signal (after labeling with an enzyme) if it stays intact (low current densities indicate a high target concentration).
We showed in 2019 that we could achieve a picomolar range sensitivity within four hours and, after the pandemic hit, we decided to adapt and improve our system for COVID-19-specific genes. There, we shortened the assay time and the whole sample-to-result time to 30 minutes. We could detect about 2000 to 8000 copies per microliter, depending on the gene, without any nucleic-acid amplification. However, it is still not automated, so we have some dispensing steps and manual pipetting. This will be one of our future works to create a fully-integrated detection system.
With our CRISPR-Biosensor, we can measure different genes (viral RNAs – theoretically any kind of RNAs and DNAs by using different Cas enzymes) at the same time or bring in other bioassays such as the antibiotic assay we developed for gauging beta-lactams. In this study, one of our main goals was to demonstrate that our device is flexible, and we can bring any kind of bioassay (here, a protein-based assay for antibiotics) with a CRISPR-based assay (in this work, for COVID-19 detection) together.
Another interesting device your lab built is a lab-on-a-chip biosensor that can determine antibiotic levels from breath samples. Can you tell us about this?
It is actually the same lab-on-a-chip device. Both systems are just electrochemical microfluidic biosensors. For one we are using CRISPR for nucleic acid detection and for the other we are using the penicillin-binding protein of bacteria, which is resistant to beta-lactams stemming from its cell membrane. The good thing is this protein is unspecific for all beta-lactams and recognizes the beta-lactam ring in general, which is better than using antibodies because there you have to make a specific one for each type of beta-lactam.
With this assay, we are capable of measuring below the nanogram per milliliter range (196 pg/ml), which enabled us to measure breath samples. In a pig study, we were capable of not only detecting traces of the antibiotics, but we also saw a simultaneous drug clearance behavior same as in the blood-based samples.
I believe breath analysis is very promising because a very good exchange with the blood for many analytes exists, and also very rapidly because there is high circulation. We are now making a follow-up study using other antibiotics and also animals with lung injuries to see if the correlation is still there.
For many lung diseases, you apply drugs through inhalation, so it will be interesting to have a sensor-integrated system (for example, using e-vapes) capable of measuring the drug concentration from exhaled breath and regulating the drug concentration in a closed-loop manner. In general, we would like to be able to efficiently adjust the dosage of an antibiotic by increasing the dose or decreasing the time of the application of the antibiotic. Everyone has different physiology and could clear the drug faster or slower, so our goal is a personalized antibiotic therapy.
What future problem or challenges do you hope your group can one day solve?
Today, we are living in a population-centered care model, for example, where a drug dosage you receive is tested on a small population. It is not just for you. I would like to use sensors which look at your drug clearance behavior and personalize the drug dosage for you. This can also be expanded for specific patient groups, like COPD patients who cannot breathe well. You can design masks which are tailored for this group and also tailor their drug dosages and/or measure their health status at home.
I also want to use the power of the data from sensors (especially wearable ones) to offer proactive instead of reactive care. We can see a change in a patient’s blood, sweat, or breath parameters over time and then predict future diseases. I would like to involve users and patients very early and then proactively eliminate the diseases or at least offer a very early diagnosis.
You have a YouTube channel called “Disposable Microsystems” where you discuss your research and other scientific topics. Why did you start the channel and what do you hope to achieve
I believe science is about sharing information and, of course, YouTube is one of the channels that we are using to accomplish that. I am trying to be very active on Twitter, LinkedIn, Facebook, and Instagram as well. In each channel you have different audiences and there are some countries, for example, with restrictions from using Twitter, but you can reach them on LinkedIn or on Facebook.
There are also age groups which use more of one social media platform over others. The idea is really to reach as many people as possible. Everyone does excellent research, but you cannot wait for people to find your research. When you post something on Twitter, for example, you have maybe less than a second to catch one person and attract them to your work as they are scrolling through their feed. Being visual is the easiest way to do this.
Regarding YouTube, I try to upload some of my talks without worrying about how the video is used because the talks change over time, and I always explain things differently. I even have a talk about the connection between social media and science promotion and I have heard a lot of positive feedback. Things are moving slowly, but you need to start somewhere.
You have been a principal investigator for a few years now. How do you approach your role as a supervisor and is there any advice you can give other principal investigators who are just starting out?
It is important to understand that every student is different, so you need to approach each student differently. You cannot have a common approach for everyone.
My approach is to study my students and learn what they are expecting from me and we try to create a healthy and productive situation. If someone would like to write papers, I give them chances to do that. If someone would like to focus on just writing the minimum required, then that is also fine for me. I give my students as much freedom as I can provide, with some structure.
For example, if you start in my lab as a Ph.D. student, you get maybe three to six months to read about the topic you were hired to work on and build your own ideas. Through that time, you also learn about the methods and get introduction to the devices that you need to use for your approach. Then I try to end this phase with a review article because after the six months, you know the field and what is published so that we can bring a new perspective to it. With this, you learn how to write a manuscript, prepare figures and do all other related work. This again prepares you for your research articles upcoming.
Many students are frustrated with the academic system and ponder leaving while still being undergraduates. What do you think could be improved in academia in order to make them want to stay?
I think that the most important thing is to give them long-term perspective. We are bad at that in Germany, and I believe it is becoming even more difficult to think long term and see oneself as a professor. In many cases, from what I have seen, there are brilliant scientists who could also make excellent professors who left academia because they were afraid of losing their jobs and not becoming professors in a reasonable time or even not at all.
I can say all of my former students found a job within a very short time and they are happily working in different companies, with one of them in academia. One of our goals is also to build very well-educated employees for companies as well as universities. I see many people would prefer to stay in academia and I think academia should find a way to integrate them into the system. Germany has a good way of doing this with permanent positions (very limited number existing), but unfortunately, they are now thinking of cutting these positions instead of extending them.
I believe if you would like to do excellent science, you need infrastructure, and it is not good if you are having a position changing every three years and you need to hire new staff. I think you need to be more open for permanent positions and maybe save money from other sources. This would be a good way in academia to integrate many people who are not willing to be professors but want to stay in academia as researchers.
If you want to stay in academia, you need to fight a little bit harder, and there were times when I thought I could not make it, but I always decided to fight back. For example, I have been rejected by many different early career researcher programs to build my independent research group, however, I did not give up and instead applied with these ideas to normal research programs and received the funding required to conduct research independently. If I would have known that it would take seven years and many hurdles to get where I am today, maybe I would have made different decisions, who knows.
My goal is to make this phase easier for my students and introduce them to my own connections to enhance their career. Only a few people helped me get to where I am now, so I know how difficult it can be and I do not want my students to face a similar situation. This is of course frustrating but social media can help make the public aware of the problems.
What is a hobby you are pursuing?
I like to play and watch football, and support the teams: SC Freiburg in Germany and Besiktas in Türkiye.
A good read you can recommend?
I really like the work of Jack London or any books explaining history coupled to a fictional story. This is much more interesting than just a history textbook.
A discovery (any research field or time) you wish you would have made?
Together with Allen J. Bard, I would have liked to discover the first biosensor for glucose measurement. I also would have liked to help Eva Engvall and Peter Perlmann discover ELISA [enzyme-linked immunosorbent assay to detect and measure the concentration of an analyte, for example, using antibodies directed against a surface protein of a virus which can be measured]. I really like their approach because it makes me think of engineering. They combined different advances in histochemistry, serology, radioimmunoassay and optical spectrometry to create a methodology which is successful even today mainly due to its easiness.
A person (famous or not) you would like to meet?
I think for this question, I am going back to my roots. I originally come from Türkiye, so I really would like to meet Mustafa Kemal Atatürk, the founder of the country. I find him fascinating, because he decided, in the 1920s, in a country like Türkiye, which is predominately Muslim, to give women the right to elect and be elected to the parliament and transformed the country into a secular republic.
As a scientist, I really like his approach (“Our true mentor in life is science”) from so long ago to follow the science as it evolves, not his words or his beliefs at the time. Even today, many world leaders cannot grasp the idea to follow the science, particularly because the facts of science will change as we look forward to better understanding and better studies.
In science, theories need to be able to shift or be rejected in line with changing evidence, and communicating this on the internet is a massive challenge. As an example, when we published the article about the first facemask-integrated with paper-based breath sensors, it was making news exactly after the first wave of COVID infections started. There was a message as a response to a tweet from the university that someone wrote sarcastically, saying we really picked a good time to publish this paper, as if we have something to do with the COVID pandemic.
I answered him that we published this study over a year ago and showed him the original article and that the media attention coming at the same time as COVID was just a coincidence. Then he started directly communicating with me and thanked me for my response and asked if we also would like to use this device for other diseases. That is a great example of how you can stop miscommunication before it gets worse just by directly pointing out the facts.
Who would play you in a biopic?
James Spader from The Blacklist would be a great choice. I really like him and that show.

















