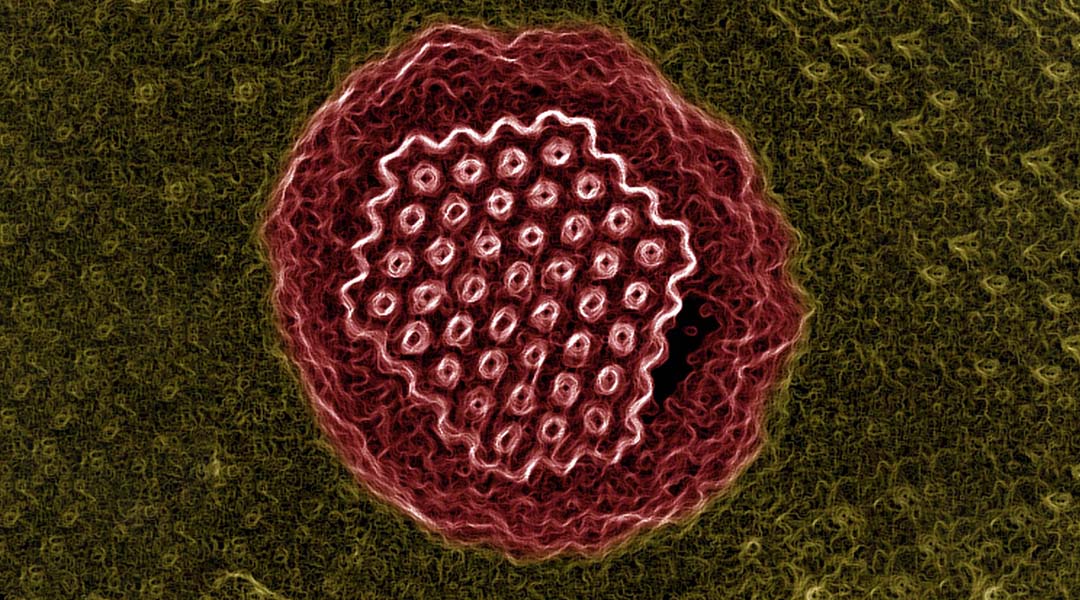
Recently, scientists have reported the discovery of an almost 50,000-year-old virus in the Siberian permafrost — and they were able to revive it. Permafrost deep in the heart of Russia offers the perfect environment for these viruses to lie inactive for tens of thousands of years.
This month, a team of researchers from France, Germany and Russia led by Jean-Michel Claverie, emeritus professor at Aix Marseille University, published research in which seven of these zombie viruses, which range in age from 27,000 to 48,500 years, have been revived. The term “zombie” has been used to describe these viruses because of their ability to seemingly come back to life when rescued from the permafrost. In actuality, they were never dead, just dormant.
This work has raised concerns about future outbreaks as a result of these ancient viruses. However, Claverie says that his work poses negligible risk because he is specifically working with pathogens that cannot infect humans. However, the real threat he says, is human- or animal-infecting viruses, those we thought extinct or never knew existed and which are still lurking in the frozen soils, particularly with global warming accelerating the melting of permafrost around the North pole.
With a background in biophysics, cell biology, and bioinformatics, Claverie and his team are working to unlock the secrets of these newly discovered ancient viruses.
What are zombie viruses and what fascinates you about them?
I did not invent the term zombie viruses. It was invented by the media when we discovered the first viruses capable of being revived after a long time in the permafrost, called Mollivirus and Pithovirus, in 2014 and 2015. The media went totally crazy about this when we published the paper and started calling them zombie viruses. Now, I use the term because I find it very catchy.
What is fascinating is we could have an infectious continuity with something that eventually could have caused the extinction of Neanderthals still alive in permafrost. This extinction happened about 50,000 years ago, and we know they lived around in Siberia at that time.
The WHO is happy to say they have eradicated the smallpox virus, but really that might not be true because of permafrost and mummification. Many people have been mummified and buried after they died from smallpox and in those mummies, you could very easily find the sequences of the smallpox virus. Fortunately, no one has tried to revive that, but we know there are plenty of viruses still infectious in those types of settings.
This is why we confine our research to amoeba viruses [viruses that infect amoebas, single-celled microbes that usually live in aquatic environments], because we want to be very safe. Amoeba do not share any viruses with animals and humans because of their extremely large evolutionary distance.
How do these ancient viruses compare to contemporary viruses? Do any from the current study have modern descendants that we would recognize?
There are different families of amoeba viruses and they have been around for close to one billion years because they are very contemporary to the emergence of eukaryotes (i.e., type of cells with nucleus, like ours and those of animals and plants).
For them, 10,000 years or even 50,000 years is nothing. Those viruses are very similar to what we have today, about 93% identical in terms of nucleotide sequences, so they do not mutate very much. What makes viruses mutate is the fact that they are confronted by defense mechanisms, like the human immune system. There is then a permanent war between the virus and its host.
However, for a protozoan [single-celled organism such as an amoeba] and all planktonic life in the ocean, the best defense against a virus infection is to die quickly so you do not propagate the virus to the rest of the population. Because of that, the virus is not confronted with a defense it has to circumvent. They just kill the host and stay there waiting for another host, so there is no real incentive to mutate or to try to penetrate defenses that change constantly.
Thus, we can then recognize ancient virus by comparing them with their modern counterparts from the same families. They could share as much as between 85% to 90% identity at the nucleotide level.
How many viruses have you discovered to date?
Now, we have discovered 15 viruses, but we really have many more in the pipeline. It takes quite a long time to characterize a new virus if you want to do it exhaustively. For each virus, we want to publish the genome sequence, the transcriptome, the proteome, and the complete replication cycle seen by various techniques of electron microscopy. We are not going to do that for 15 viruses, especially when there are 90% identical to the previous one.
That is why the very last paper we published that started the whole buzz is just an update. We wanted to say that having viruses survive in permafrost is not surprising. Almost every time we try to isolate a virus from any kind of sample, we do so successfully, but we do not have time to work them out in detail because we are a small lab of about 20 people.
Where do you and your team recover the viruses from and how are they extracted?
Our place of play is Northeastern Siberia because this is where most permafrost is found. We have our Russian collaborators send us samples by mail or bring the samples to us. Recently, I wanted to go myself to a sampling point because I think that when you are doing this kind of research, you really need to appreciate how it’s done, especially to build your confidence about the possibility of contamination or how those things are handled correctly.
I went to Siberia for about one month in the summer of 2019 to Yakutsk, the coldest city on Earth, but it was 30 degrees Celsius in the summer. Then I went to Cherskyi, one of the most eastern cities in Siberia that is close to some places that have been extremely well studied called exposures — banks of rivers where heavy erosion takes place. You have cliffs, which are very nice tools because, as you go deeper, the permafrost is older.
Normally, if you want to reach deep layers you have to go through the younger layers first and then you contaminate the older permafrost. With cliffs, you simply remove all the soft material until you get to something that is concrete and solid and, because permafrost is extremely hard, there is no ambiguity. Then you start drilling horizontally and you go directly to the level of the age you want to study. You can also recognize prehistorical squirrel nests, which are great samples because they are full of seed remains.
The layers are already dated by the geologists, and this is really nice because you totally abolished the problem of contamination by modern materials. You can very accurately find, for example, remains of mammoths or woolly rhinos in the permafrost layers and take your sample from those remains directly.
Your team recently revived a 48,500-year-old virus from the Siberian permafrost, making it the oldest virus ever found. Why is this discovery exciting and what makes it so important?
This is going to be taken as some kind of a limit but, of course, the limit is only the technical limit of radiocarbon dating. We cannot go much beyond that, but I am sure there are viruses that can stay infectious after 100,000 or 200,000 years. This would be more difficult to demonstrate because then you have to use a more sophisticated dating technique that we do not have in the lab and that are trickier to use.
What is exciting about this is our ability to demonstrate that the discovery of the first two viruses that we were able to revive was not a lucky shot or just something that will never be reproduced. We know now that every time we are going to look for viruses in any kind of permafrost sample, we will probably find some. What is more important is that if amoeba viruses can be revived after that long, there is absolutely no reason why other viruses could not also be revived after that many years, including viruses that can infect animals, plants, or humans.
We use those amoeba viruses as surrogates for the real thing. We must be careful when you do research in permafrost because there might be some surprises for you, including viruses we thought had become extinct. Global warming is accelerating the release of those microbes from ancient layers of permafrost and also making those regions accessible to human and to industrial adventures. Before global warming, you could not bring equipment by boat because of the ice cap, but now you can do this almost eight months a year. You can bring all the equipment that is necessary to do mining, for example.
Open pit mining is basically first removing about 500 to a kilometer deep of permafrost because permafrost does not contain anything worthwhile. The real soil beneath that is full of minerals, gold, and diamonds, or precious metals. You remove millions of tons of permafrost, which can be up to a million years old, and which we know absolutely nothing about. We do not know what kind of bacteria are there or what kind of viruses are there because we only know of viruses up to 50,000 years old.
Also, this mining is not done in a [Biosafety Level-4 (BSL-4) facility, which is a laboratory that provides the top level of security, allowing scientists to handle pathogens of the highest risk]. This is open pit mining, which is extremely polluting, and you do that with workers who are in fairly confined living quarters. If something happened and one of those workers catches a very old, nasty virus from a Neanderthal in the permafrost, this will probably get into the colony very quickly and those colonies can be big because the Russians have developed the technology of floating nuclear power plants. They can bring those nuclear power plants anywhere on the Arctic coast and create a city and have it supply energy for up to 100,000 people with one plant.
We encourage those companies to have a competent medical facility with people knowledgeable in infectious diseases, eventually capable of recognizing strange symptoms, and instead of putting those sick people immediately into a plane back home, maybe have a place to quarantine them and have enough equipment to do the relevant medical testing on site. Unfortunately, I do not think that anybody is doing that.
What about these viruses allows them to be preserved for so long?
What kills viruses in the open air is UV light, oxygen that oxidizes proteins and nucleic acids, heat, and extreme pH levels, like highly acidic or highly basic environments. Permafrost is the exact contrary of that because it is cold, anoxic (no oxygen), dark (no light), and is pH neutral in Siberia. This is the ideal place to keep organic matter forever. I am sure if you take a yogurt and you put it in permafrost and come back 50,000 years later, it will probably be still good to eat.
Viruses, as particles, do not require any metabolic activity to maintain their structure, so in absence of any perturbation, they will stay like that forever. The only thing that could have some kind of an effect would be natural radioactivity, which could very slowly degrade DNA, but many of those viruses also have a DNA repair mechanism. The very first thing they do when they get into a cell is to repair their DNA before they start transcribing it and synthesizing their proteins.
The only reason I think we became the demonstrators of this fact is that nobody wants to take the risk of working with viruses in permafrost because there is a real risk of reviving something nasty. We had luck in choosing the right system by using the right host, the acanthamoeba, which is [found] in humid environments and is easy to cultivate in the lab.
With this type of amoeba, we can do virology in a BSL-2 facility. In fact, this level of biosafety is not required by the virus, but because these amoebae (acanthamoeba) can be slightly pathogenic and can give you keratitis, for example. For us as researchers, the (slight) danger comes from the host [the amoeba], not from the virus itself!
What is the procedure of “reviving” these viruses?
We first cultivate amoebas in petri dishes. We take a small amount of the permafrost sample, normally a gram, and put it in the petri dish and wait to see what happens. Quite often, these amoebas become unhealthy and start dying, meaning something is killing them.
Amoeba naturally feed on bacteria, so bacteria are usually not the problem. Microfungi could be a problem for contamination because there are some live microfungi in permafrost too. However, most often when we have a dying amoeba, it is because a virus infects the culture, and multiplies while killing the amoeba.
After multiple passages, we get millions of virus particles that we can see under a microscope. This is another advantage of our system: most amoeba-infecting viruses happen to be giant, so a light microscope is sufficient to detect them while most other viruses require an electron microscope.
This technique is called co-cultivation. We learned this when we were working with the parasitic bacteria, Rickettsia, that also multiply within acanthamoeba. The very first virus we discovered in 2003, the Mimivirus, had been spotted about 12 years earlier but mistaken for a bacterium. We finally realized this was actually a virus despite the large size of its particle, typical of that of a small bacterium.
Many argue the ethics and dangers around studying viruses such as these. In your opinion, why is this work important to do? What questions are you trying to answer?
We wanted to show that viruses can still be infectious after spending a long time in permafrost, and I believe we have succeeded in demonstrating that. Now, we are turning back to more basic research to study those viruses in detail using genomics and transcriptomics, as well as using genetic approaches that were recently designed in our lab.
This means that we can target specific virus genes and make mutants. What characterizes these viruses is that they have many genes that do not resemble other genes in any other microbes or cells, called “orphan genes”. Making mutants is thus a great way to investigate their function. We hope to eventually solve the mystery of the evolutionary origin of these viruses and how they compared to others. We already have 50 viruses that need to be fully characterized and investigated, so we have plenty of work for the century to come.
Another question I am also concerned with is a totally different story. I am part of what is has been called the Paris Group. We are a group of French virologists that believe that COVID is not a classic case of zoonosis [viral transmission between animals to humans] but in fact probably came from a lab accident in Wuhan. I am not talking about viruses being engineered or even created in the lab, but simply people going into places like caves, trying to see what kind of viruses could infect bats. Then they bring back many samples, including blood, feces, and even the live animals. It is perfectly possible that somebody got infected at the early stage of characterizing viruses from the environment because at that point, there is no reason to work into a high biosafety level lab. You just try to grow a bat virus from your sample, without knowing if it might be able to infect human cells.
In my professional opinion, if it is not a very highly symptomatic disease, like COVID, someone can come back to their family and that family is going to the market and that becomes the first location for amplification of the virus. The origin is probably not an animal that was sold in the market. The coincidence is just too great to see a pandemic starting in the city of Wuhan that is also the location of several top-level research institutes on coronaviruses.
So yes, [I believe] there is a real danger in doing that kind of work, especially when they try to identify what could be the next viruses capable of infecting humans. Not only will they try to infect human cells in vitro (or using “humanized” mice) using those unknown viruses but even worse, they may try to see which gene might mutate and make these viruses more infectious/lethal to humans. That is called “gain of function” research.
After a given point, I am not sure you really need to know more than what we know, especially sending people to places where they would never go normally, including the deep Amazonian rainforest and caves in China, just to see what is there. This is the dark side of the trendy “one global health” projects. You really have to be very careful what you are doing and eventually we will need some kind of a moratorium on this kind of work, especially concerning human-infecting viruses.
In fact, we do not really need that in the sense that using metagenomics, we can see what kind of viruses there are without taking the risk of reviving them. We already do that all the time to verify that the virus that was isolated is really coming from the sample and is not a contamination. When you do that, you realize that the permafrost contains a lot of viruses which probably could infect organisms that are close to humans, such as asfarviruses, herpesviruses, adenoviruses, and poxviruses.
What are next steps or future plans?
With [viral mutations], we are knocking out genes [a genetic technique in which one of an organism’s genes is made inoperative to see its effects] and we already have some very interesting results. We can see that in those very big viruses, like the pandoravirus, you can remove up to a third of the genome and nothing happens. This goes very much against the idea that viruses are extremely well optimized.
I think personally, because I am officially retired now as an emeritus professor, I want to investigate new concepts that might explain the evolution and the forces behind the evolution of those giant viruses because they do not obey the classical neo-Darwinian concepts. For instance, they seem to be able to create new genes (and hence new proteins) out of non-coding DNA. Thus, not all genes have an ancestor, contradicting today’s evolutionary paradigm.
Why the heck would a virus have 2500 genes? What are the evolutionary incentives for becoming such a thing? I can see a parasite that is initially big and complex becoming smaller and smaller and less complex, but not the other way around. I am not satisfied with the with various current hypotheses about it so I will probably work more in those areas in the future.
Feature image: Pithovirus sibericum. Credit: © IGS – CNRS/AMU