 Calcium phosphates (CaP) based materials, specifically hydroxyapatite (HA) and tricalcium phosphate (TCP), are extensively used as synthetic bone graft substitutes for various applications (e.g. bone augmentation procedure) or as biomaterials for in vitro evaluation (e.g., in vitro bone tissue models).
Calcium phosphates (CaP) based materials, specifically hydroxyapatite (HA) and tricalcium phosphate (TCP), are extensively used as synthetic bone graft substitutes for various applications (e.g. bone augmentation procedure) or as biomaterials for in vitro evaluation (e.g., in vitro bone tissue models).
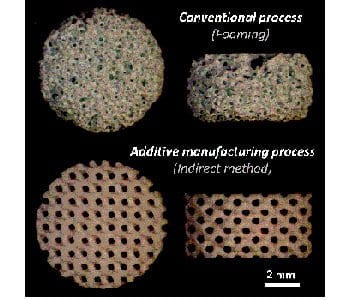
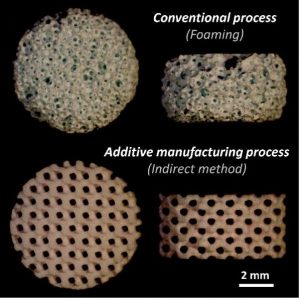
Until now, these bioceramics have been mainly produced with traditional ceramic processes (e.g. porogen leaching, phase separation, gas foaming, replication of PMMA template, bone machining). These conventional methods suffer from architectural limitations, internal inhomogeneity and sample-to-sample variations. This lack of architectural standardization prevents the rigorous design of biological experiments, their reproducibility, etc., which may lead to misinterpretations.
New engineering developments combining computational methods and additive manufacturing (AM) technologies are able to overcome such limitations by providing a higher level of control over the design of manufactured scaffolds with the opportunity to create optimized custom architectures in a reproducible way.
However, the “direct” AM technologies, such as ceramic ink writing, stereolithography, or selective laser sintering and melting, have still major drawbacks such as their lack of accuracy and flexibility from one bioceramic phase composition to another, the changes in the chemical and phase compositions (especially for CaP), the post-processing issues (e.g. the removal of raw materials), and their cost. A reliable alternative may be found in the ‘’indirect’’ AM methods, where the outstanding potential of ‘’direct’’ AM technologies for polymers is combined to traditional ceramic impregnation processes. Although the “indirect” AM method is not new, it is still relevant compare to “direct” methods provided that some technical challenges are dealt with properly.
In this context, researchers from Center for Health Engineering (Saint-Étienne, France) optimized then evaluated the reliability and robustness of a bioceramic manufacturing process based on the impregnation of 3D-printed mold, both studies are presented in the Journal of the European Ceramic Society and Advanced Engineering Materials, respectively. The developed process offers new architectural opportunities, including architecture standardization, high and reproducible architectural control, phase biocompatibility preservation and ease of implementation for various phase compositions.
This manufacturing process has allowed the fabrication of CaP bioceramics for in vitro and in vivo (e.g., critical and non-critical size, rat model) experiments with our partner laboratories about problematics dealing with the influence of the scaffold architecture on angiogenesis and osteogenesis, the influence of the scaffold chemistry on cell fate, or the origin of the bone regeneration; works which will be soon available in peer-reviewed journals.