 The properties of materials degrade at high temperature, and the corrosion of structural components such as boilers, gas turbines and tubes is a significant problem. The high operating temperatures experienced by such components significantly lowers their durability due to enhanced corrosion rates; however, increasing the working temperature is desired to increase the efficiency within industrial plants. The state of the art materials employed limits the working temperature and simultaneously lowers the service life of the components.
The properties of materials degrade at high temperature, and the corrosion of structural components such as boilers, gas turbines and tubes is a significant problem. The high operating temperatures experienced by such components significantly lowers their durability due to enhanced corrosion rates; however, increasing the working temperature is desired to increase the efficiency within industrial plants. The state of the art materials employed limits the working temperature and simultaneously lowers the service life of the components.
Newly developed high entropy alloys (HEAs) comprised of multi-principal elements are a potential candidate for use in high temperature environment owing to their sluggish diffusion behavior. However, detailed comparative studies are required before HEAs could be used for real life applications.
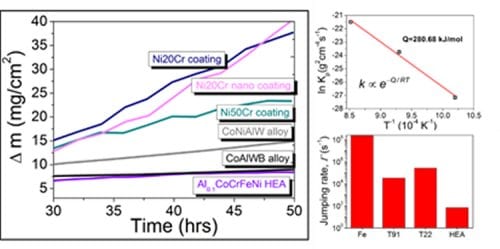
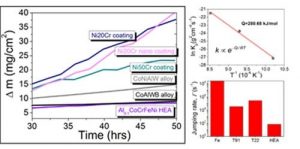
Recently, the oxidation behavior of Al0.1CoCrFeNi HEA was investigated at different temperatures (700 to 900 °C) and compared with the state-of-the-art materials. The HEA demonstrated significantly lower oxidation compared to conventionally used steels and Ni-Cr coatings. The oxidation resistance of the HEA was due to the high activation energy required for the diffusion of oxygen and high configurational entropy. The high activation energy increases the energy barrier for the atomic movement. Further, high configurational entropy entraps the atoms in the lowest potential energy sites, restricting further motion. Together, this results in a sluggish diffusion of oxygen in HEA enhancing its oxidation resistance. The sluggish diffusion also limits the thickness of the protective oxide layer increasing the stability.