Pharmaceutical targets of G protein‐coupled receptors (GPCRs) are overwhelmingly represented among prescribed drugs, as these receptors are involved in multiple signaling pathways of essential physiological stimuli such as light, peptides, neurotransmitters, hormones, lipids, and ions. GPCRs can be grouped into five major classes based on sequence conservation in their transmembrane domain, among which the Class A of these receptors is the most abundant subfamily. Receptors from this subfamily bind bioactive lipids that have vital physiological roles, and are therefore extremely valuable therapeutically.
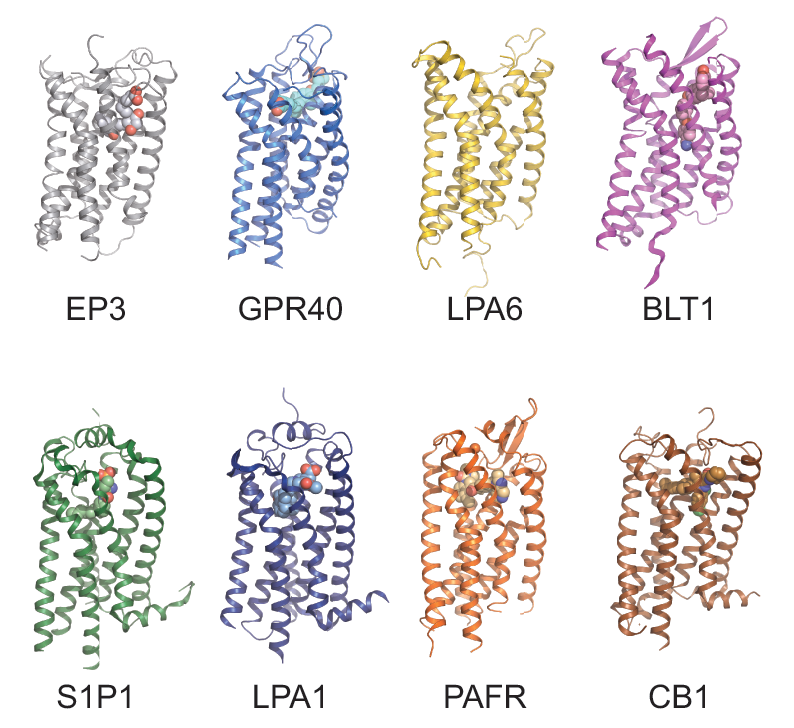
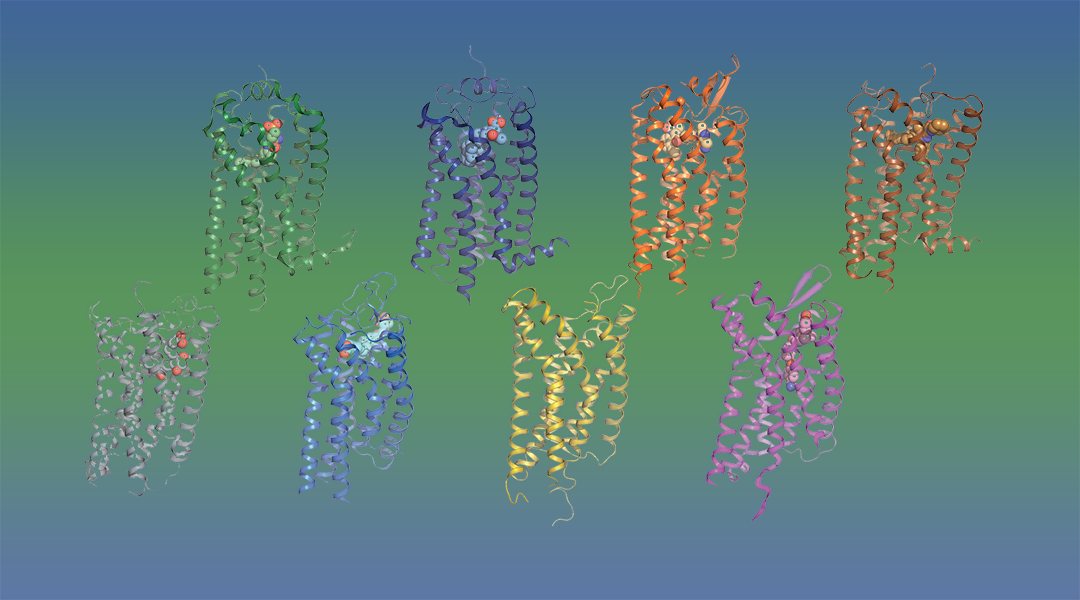
Eight unique lipid-binding receptors. Prostaglandin E2 receptor 3 (EP3); free fatty acid receptor 1 (GPR40); lysophosphatidic acid receptor 6 (LPA6); leukotriene B4 receptor 1 (BLT1); sphingosine‐1‐phosphate receptor 1 (S1P1); lysophosphatidic acid receptor 1 (LPA1); platelet‐activating factor receptor (PAFR); cannabinoid receptor 1 (CB1)
However, developing drugs that target GPCRs has been a continuing struggle, largely due to biochemical and pharmacological hurdles such as the instability of the receptors, particularly in the active state, making the determination of the structure of these receptors even more difficult. Some of these hurdles have only been overcome in the last two decades using complementary modelling and experimental approaches.
Given the hurdles due to pharmacological properties, resolving the tridimensional structures of lipid of receptors using high-resolution using X‐ray crystallography has proven to be an essential and complementary approach to grow our understanding on mechanisms of action, and to design various ligands.
The unresolved structural and pharmacological characteristics of lipid receptors that arose from the most recently identified structures of eight unique lipid-binding receptors that differ from other GPCRs by having an obstructed ligand entry site, are discussed by Audet and Stevens in Protein Science. The few available structures of lipid‐binding receptors unveil a promiscuity of lipid action due to a higher binding site plasticity, and explain the atypical pharmacology of some lipids.
These studies not only offer impactful mechanistic insights on the fundamental role of GPCRs in the physiological integration of the lipid signaling system, but also stress the importance of continued research into the structural biology of GPCRs for the rational design and development of new therapeutics targeting lipid receptors.
Written by Marina Ostankovitch.