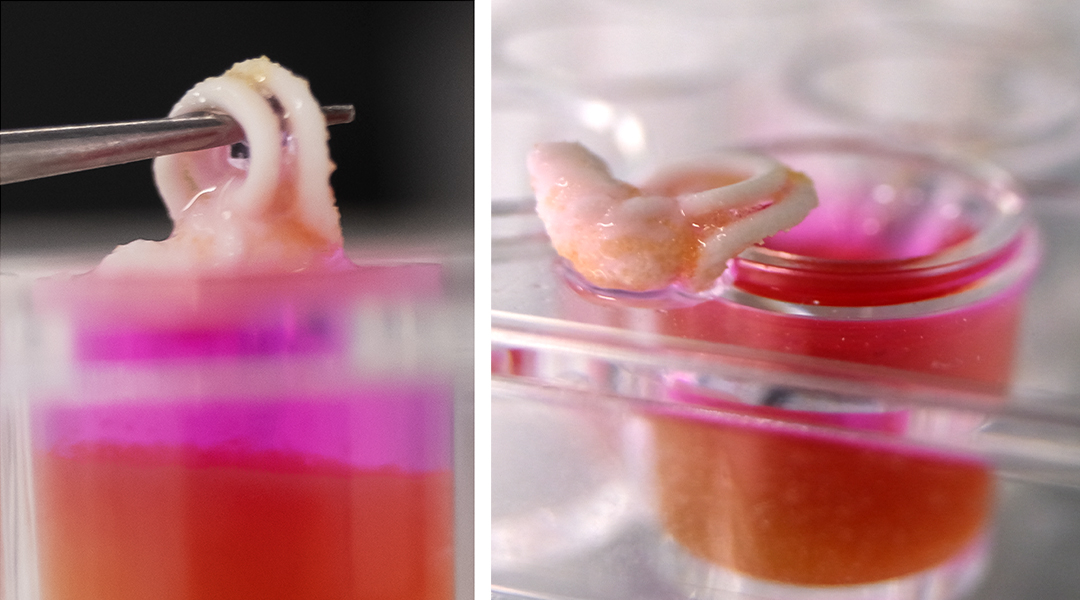
Reproduction of human inner ear bones; supplied by Sara Romanazzo
Imagine surgeons simply printing new bone right there in the operating theater to repair tissue damaged in accidents or by disease. This capability is now a step closer thanks to a new 3D-bioprinting technique developed by researchers from the University of New South Wales, Sydney.
As reported in Advanced Functional Materials, the research team led by Professor Kristopher Kilian and Dr. Iman Roohani use a novel calcium phosphate-based ceramic ink that sets at room temperature within minutes in aqueous environments.
Up until now, attempts to fabricate bone replacements by 3D-printing have mostly relied on very high temperatures or strong chemicals to harden the products — factors that are incompatible with incorporating live cells or sensitive biomolecules.
“Our technique solves these problems by allowing construction under mild conditions in the presence of biological molecules and cells, without heating or toxic chemicals, while at the same time affording structures with bone-like architecture and mechanics,” said Kilian.
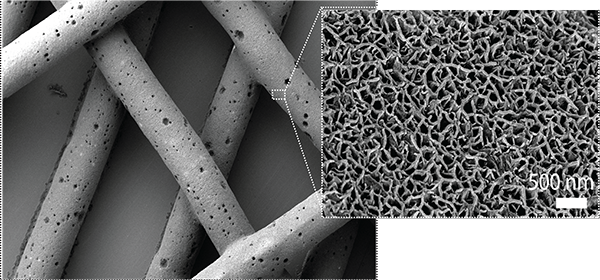
The 3D-printing technique is called ceramic omnidirectional bioprinting in cell suspensions, or COBICS. The ink is extruded into a mixture of microscopic hydrogel particles and live cells, which allows high-resolution, free-form printing of complex constructs, such as the tiny bones that are a part of the mammalian inner ear.
“The support bath behaves as a solid until the ceramic ink shears into it at a certain force,” explained Kilian. Live bone progenitor cells in the bath then migrate onto the printed scaffold to build new bone around the ink.
“Stem cells in the bath immediately attach to the printed structure, then they proliferate and begin to turn into bone cells just by virtue of the material. This more closely emulates natural bone formation processes,” said Kilian.
“The ink takes advantage of a setting mechanism through the local nano-crystallisation of its components in aqueous environments, converting the inorganic ink to mechanically interlocked bone apatite nanocrystals,” explained Roohani.
Currently, surgeons use bone grafts harvested from another part of the patient’s body to repair large areas of bone tissue damage and defects. The success of this approach is limited by being able to find suitable bone tissue, morbidity at the harvest site, and risks of infection.
“We believe the integration of fast setting ceramic ink which has a similar chemistry to native bone minerals with printing in cell suspensions is transformational to bone tissue engineering’” Roohani said.
With the printed material taking only 5–10 minutes to harden in the presence of water or bodily fluids, the authors envisage that one day doctors could print “bone” implants with the patients’ own stem cells incorporated into the ink, or even print directly into the body where there are resident stem cells.
With trials starting in animals, the team are setting out to investigate whether their 3D-printed bone mimics will integrate with existing native bone.
Reference: Sara Romanazzo, et al. Synthetic Bone-Like Structures Through Omnidirectional Ceramic Bioprinting in Cell Suspensions, Advanced Functional Materials (2021). DOI: 10.1002/adfm.202008216