Targeted delivery of anti-cancer drugs and diagnostic agents to tumors is the holy grail of cancer medicines. This is because targeted delivery could maximize the accumulation of drugs to the targeted tumor while minimizing the accumulation of the drug at any non-target organs, leading the way for successful cancer treatment without the toxic side effects or accurate diagnosis without false readings.
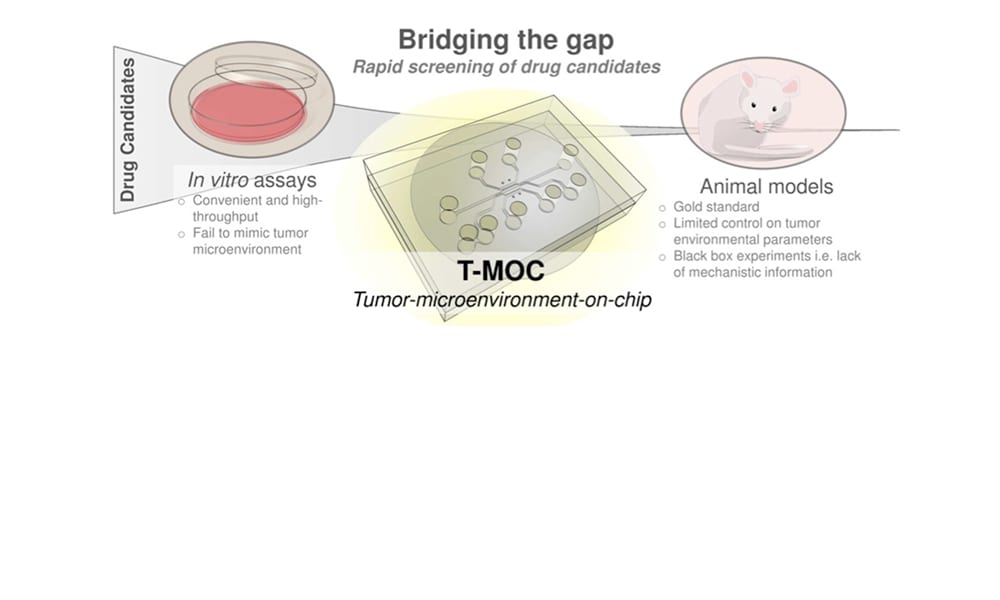
Recent development of nanotechnology enables a wide variety of nanostructures and materials as delivery vehicles, whose properties can be tailored for targeted delivery. Although this so-called “nanomedicine” has led to the success of a few new drugs such as Abraxane® (based on nano-albumin particles) and Doxil® (PEGylated liposome nanoparticle formulation), translation of many new nanostructures to clinical success has been significantly hampered. Many nanoparticle systems show promising results during tests using simple cell culture models and small animal models, yet their clinical success has been less than anticipated. This gap between preclinical and clinical studies suggests the presence of complex drug transport mechanisms at human tumors, which are missing in preclinical cell culture models. This challenge warrants a critical need for improved preclinical evaluation techniques that rapidly test the performance of new nanomedicine formulations under conditions more closely mimicking the human tumor environment.
In this review in WIREs Nanomedicine and Nanobiotechnology, researchers from Purdue University led by Professor Bumsoo Han summarize challenges of realistic evaluation of cancer nanomedicine and focus on the recent emergence of microfluidic tumor models for improved characterization of nanomedicine performance. These models not only create a three-dimensional cellular microenvironment, but also simulate physiological cues at tumor tissues including fluid pressure and flow, oxygen and nutrient gradients by precise control of flow fields (i.e., microfluidics).
One of the primary application areas of the microfluidics-based models so far has been to increase the throughput of drug screening compared to that of small animal studies. In addition, these models can provide detailed information to optimize or revise nanoparticle designs. Moreover, it is also anticipated that drug-screening platforms uniquely tailored for individual patient physiology will be designed using microfluidics to achieve “personalized” or “precision” medicines.
Contributed by the Authors.