As global energy demand continues to grow, the need to find a carbon-neutral and sustainable energy source for future generations has become imperative. An especially attractive solution is to store solar energy in the form of chemical fuel via artificial photosynthesis to convert carbon dioxide into hydrocarbons.
To apply this photocatalysis technique in practical applications, it is useful to synthesize semiconductor photocatalysts with specific facets that induce high reactive activities and high reactive selectivity. Attempts to deliberately fabricate such materials are made more difficult by the thermodynamic growth mechanisms of the crystals. Up until now, the solution to this problem has been the selective absorption of surfactants or ions on high-energy facets to suppress the growth rate along their axes.
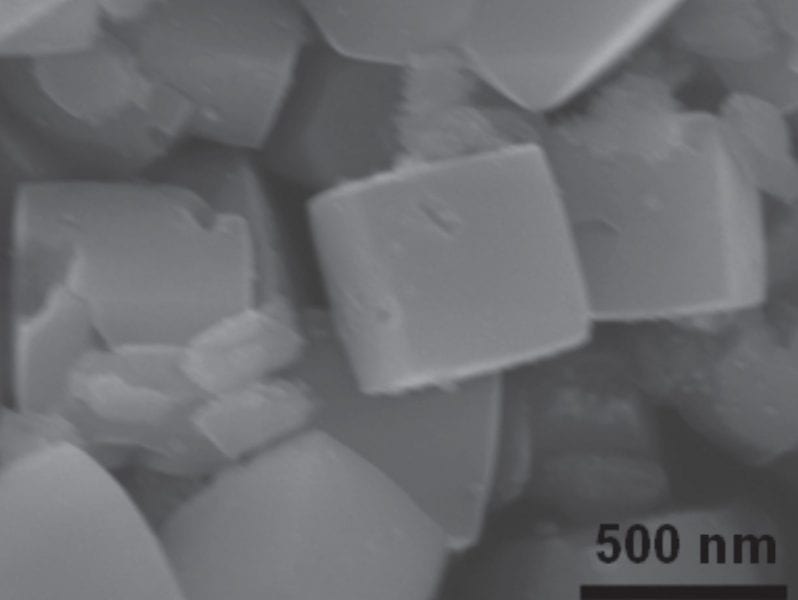
 Now, Dr. S. C. Yan and co-workers, working at Nanjing University, China, have found a simple route to synthesize ZnGa2O4 nanocubes with the {100} facets exposed. Using this ZnGa2O4 nanocube as a photocatalyst, under UV light irradiation CO2 was successfully converted into a hydrocarbon, CH4.
Now, Dr. S. C. Yan and co-workers, working at Nanjing University, China, have found a simple route to synthesize ZnGa2O4 nanocubes with the {100} facets exposed. Using this ZnGa2O4 nanocube as a photocatalyst, under UV light irradiation CO2 was successfully converted into a hydrocarbon, CH4.
Usually, for a semiconductor photocatalyst, a light-hole effective mass induces high hole mobility, which is beneficial for improving the photocatalytic reaction. However, Yan et al. used the hole effective mass to check the photocatalytic activities of a given single-crystal photocatalyst for the first time. Their work may open a novel route to find the more efficient photocatalyst for driving conversion from solar to chemical energy. A future effort is focusing on the discovery of visible-light-response photocatalyst with sufficiently light hole effective mass.