Lithium metal, which packs lithium atoms in dense volume, is the most attractive anode for high-energy lithium-ion battery. The use of lithium metal anode to significantly boost the energy density is regarded as the “Holy Grail” of the rechargeable Li-ion battery. However, strong reducing nature of lithium metal limits its application, causing incompatibility and undesired reaction with most electrolytes. The lack of long-term stability between lithium metal anode and electrolyte leads to quick battery capacity fade and cell failure. Despite decades of research efforts, the goal of stabilizing lithium metal anode over many charge-discharge cycles and long battery life time has not been fully realized.
To resolve this outstanding challenge, Yizhou Zhu, Xingfeng He, and Yifei Mo from University of Maryland, College Park perform quantum mechanical calculations powered by large-scale materials database, and uncover lithium-stable materials out of a variety of chemistry across the periodic table. In their recent article number 1600517 in Advanced Science, the authors discover that nitrides exhibit thermodynamically intrinsic stability against lithium metal. Such unique stability is lacking in other materials chemistry, such as oxides, sulfides, and halides, commonly used for lithium protection. The intrinsic resistance of nitrides against lithium reduction makes them ideal materials candidates to protect lithium anode with no degradation over long term.
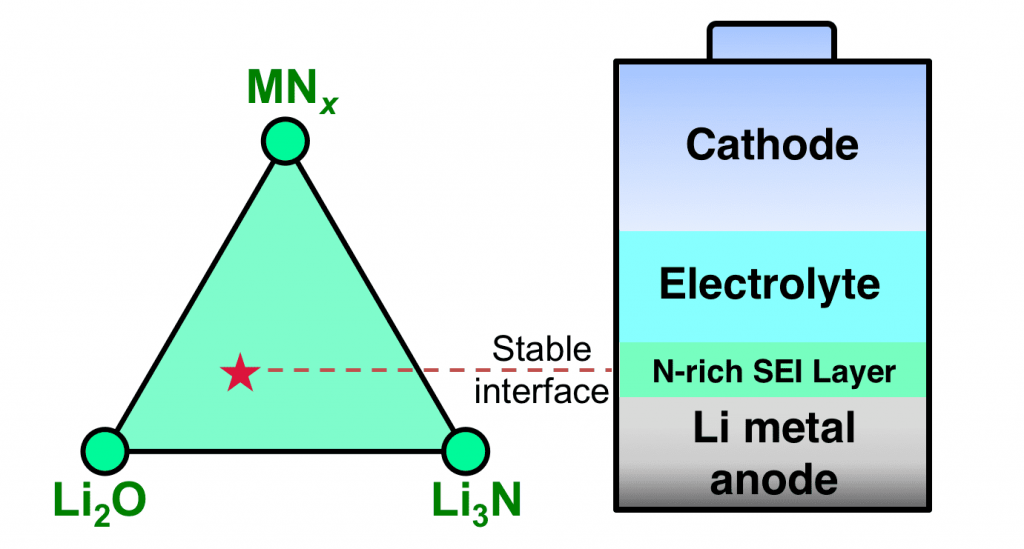
Novel stabilization methods for Li anode
This scientific discovery provides new directions for the future research and development of lithium metal batteries. Many nitrides that have not been explored are discovered as promising coating materials for lithium metal protection. In addition, the discovered chemistry suggests engineering strategies for electrolyte and interfaces to achieve lithium stability through nitrogen doping. Such nitrogen doping would lead to the formation of stable solid-electrolyte-interphase, a key phenomenon accounting for the good cyclability of commercial Li-ion batteries. With specific guidelines for selecting lithium-stable chemistry provided in this study, the ultimate solution for stabilizing lithium metal anode will be achieved in high-energy rechargeable lithium batteries.

















