Anticancer drugs that can be delivered to the tumor site provide the advantages of higher therapeutic efficiency and reduced side-effects. Therefore, the development of anticancer drug delivery systems has been progressing rapidly in the last decades. However, despite significant progress in this field, constructing an ideal delivery system remains an elusive challenge due to the fact that such a system must meet multiple requirements.
First, the system must be biocompatible, biodegradable and to allow for a selective accumulation of the drug at the tumor site. This can be done either via active targeting or passive accumulation in the tumor’s blood vessels by the means of the enhanced permeability and retention (EPR) effect.
In addition, in order to enter the cancer cells the delivery system needs to cross the endo/lysosomal barrier, which stands between the outside of the cell and the internal, cytoplasmic region. To successfully penetrate this barrier, the delivery system has to be durable enough to withstand the acidic conditions inside the lysosome, as well as the presence of the lysosomal enzymes, which often cause the degradation or deactivation of the drug.
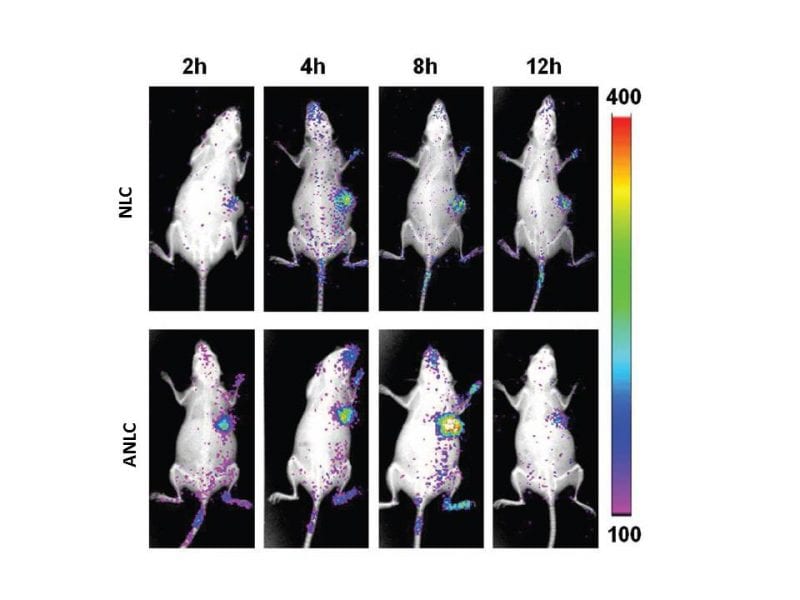
In vivo fluorescence imaging of Heps-bearing mice after i.v. injection of Cy5-loaded NLC and Cy5-loaded ANLC
Finally, the delivery system has to allow for a successful escape of the drug from the lysosome once inside the cell.
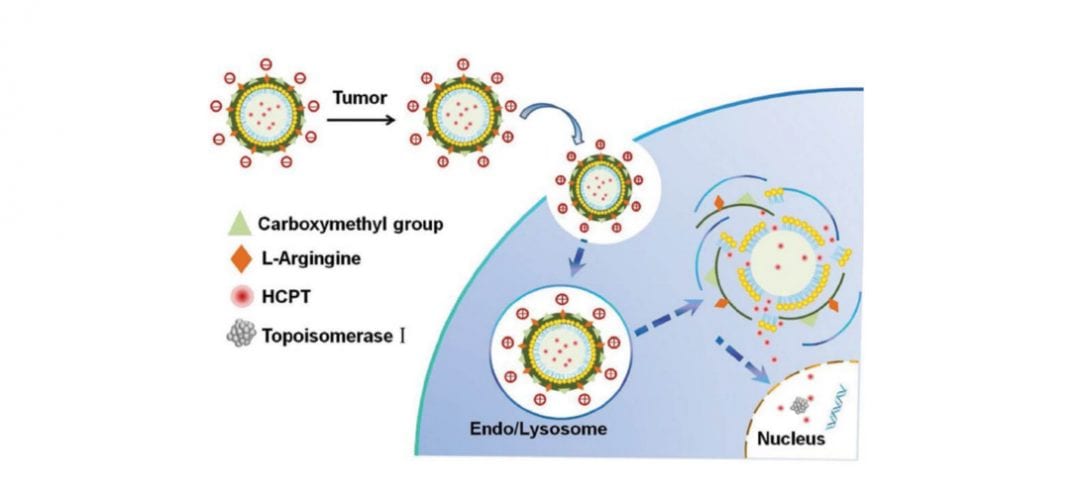
In a recent article published in Advanced Healthcare Materials, scientists have designed a pH-sensitive drug delivery system, which takes advantage of the pH difference between the lysosome and the outside of the cell. The system is based on nanostructured lipid carriers (NLC) with surface modification that allows the carriers to reverse their surface charge upon contact with lysosomal pH and to release the encapsulated anticancer drug, 10-hydroxycamptothecin (HCTP), into the cell’s cytoplasm.
These surface-modified NLCs (ANLCs) have a twofold higher cellular uptake at tumor pH compared to physiological pH, as well as a higher circulation time inside the tumor, indicative of the selectivity of the delivery system.
In addition, 63% of the encapsulated drug was released at lysosomal pH compared to only 36% at physiological pH, resulting in an enhanced growth inhibition of cancer cells.
And finally, in vivo results showed that ANLCs had enhanced accumulation inside the tumor, which severely inhibited tumor growth in mice and hence, prolonged their survival time.
Although additional research and optimization is necessary, ANLC anticancer drug delivery system seems to meet the top requirements of an ideal delivery system, and therefore shows great promise for cancer therapy.