Boron subphthalocyanines (BsubPcs) are bowl-shaped macrocycles that have recently attracted a great deal of interest due to their optophysical properties, resultin g in their use in a number of organic electronic devices such as organic field-effect transistors, organic light emitting diodes, and organic photovoltaics. Incorporation of BsubPc into a polymer could lead to preferential processing techniques and unique solid-state arrangements which could prove to be beneficial for their ultimate application.
g in their use in a number of organic electronic devices such as organic field-effect transistors, organic light emitting diodes, and organic photovoltaics. Incorporation of BsubPc into a polymer could lead to preferential processing techniques and unique solid-state arrangements which could prove to be beneficial for their ultimate application.
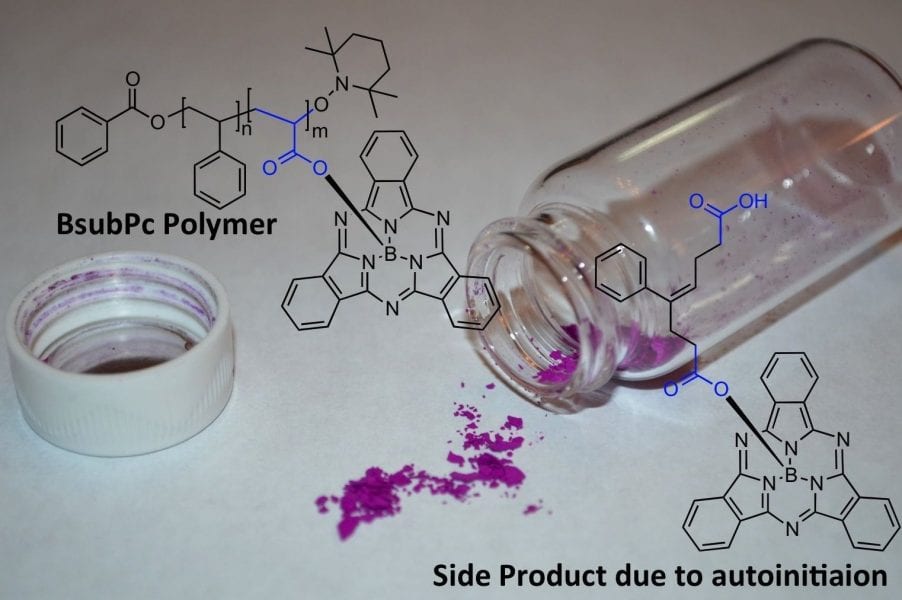
Lessard and Bender from the University of Toronto, Canada have now reported a simple and straightforward coupling reaction enabling the facile incorporation of BsubPcs pendant to a polymer backbone. The poly(acrylic acid-ran-styrene) pre-polymers were first synthesized by nitroxide mediated polymerization followed by the coupling of the BsubPc to the carboxylic acid groups on the acrylic acid monomer units.
During their work, the researchers noted the presence of a side product only after the BsubPc coupling reaction. The authors characterized this side product using mass spectroscopy and have proposed a mechanism for its formation, which they believe is a result of the auto-initiation of styrene and its reaction with acrylic acid. Because this small molecule was only noticeable once coupled to the BsubPc chromophore this would suggest that many other researchers are unknowingly producing similar side products without knowing it. This may have more general implications for styrene/acrylic acid copolymerizations.