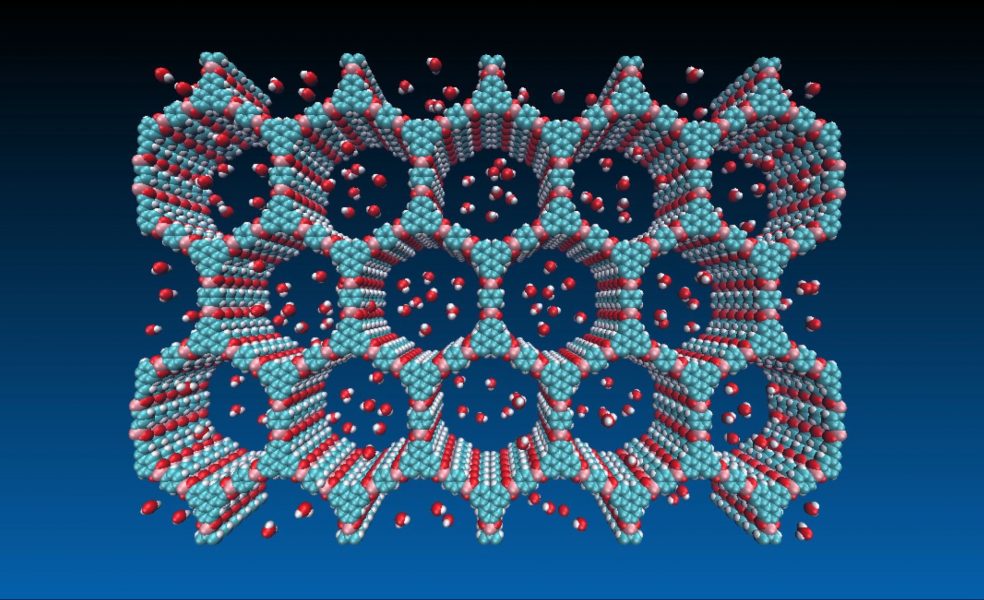
Covalent organic frameworks (COFs) represent a class of crystalline porous polymers that are formed through an elegant integration of their organic building blocks. Their high surface areas, low gravimetric densities, and nm-sized pores provide the potential for applications including gas storage, catalysis, or photo-energy conversion. For applications of these materials to be successful, however, it is important to ensure their stability.
Boronate-ester linked COFs were among the first families of COFs to be discovered and remain widely explored. However, these materials are unstable when exposed to water, which severely limits their applications. Improving their hydrolytic stabilities requires an in-depth knowledge of the molecular mechanism of hydrolysis, which is to date still poorly understood.
To contribute to this understanding, Jean-Luc Brédas and colleagues (Georgia Institute of Technology, and King Abdullah University of Science and Technology) in their paper have used density functional theory (DFT) calculations to investigate how the molecular structure of boronate-ester linked COFs evolves during hydrolysis. They identified the reaction pathways at the molecular level and found that upon hydrolysis the molecules undergo large conformation changes. Importantly, the water molecules are found to autocatalyze the hydrolysis reaction. This feature rationalizes the reason why boronate-ester linked COFs quickly dissolve when immersed in water.
The authors were also able to provide a simulation of the hydrolysis process in COF crystals; in this case, the hydrolysis reaction slows down because it relies on a different pathway. Overall, the computational results point to opportunities to enhance the hydrolytic stabilities of boronate-ester linked COFs by fine-tuning the initial molecular structure.