A simple and effective system for photocatalytic CO2 reduction is proposed
The photocatalytic reduction of CO
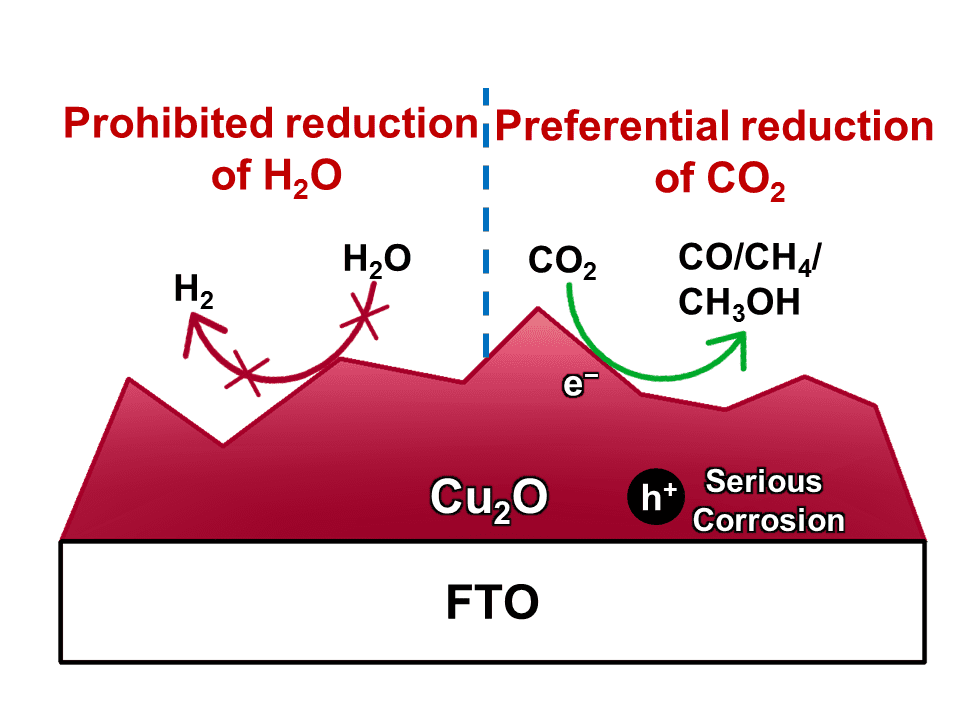
2 into chemicals and fuels is a promising way to address global warming and energy crisis. However, the hydrogen evolution reaction (HER) severely competes with CO
2 reduction for electrons, leading to a low selectivity for carbonaceous products. To suppress H
2 evolution, Cu
2O has been applied as the cathode owing to its active sites for the preferential conversion of CO
2 molecules. Unfortunately, Cu
2O suffers from poor stability since its redox potentials lay within its band gap. A common strategy to improve the stability of Cu
2O cathode is to deposit a protective layer (such as TiO
2) onto Cu
2O. But the protective layer will also prohibit the Cu
2O surface from suppression the HER, which sacrifices the selectivity for carbonaceous products.
To address this dilemma, Prof. Jinlong Gong and his colleagues at Tianjin University proposed a simple and effective system for photocatalytic CO2 reduction, using Cu2O as the dark cathode and TiO2 as a model photoanode. By shadowing the cathode from illumination, the Cu2O surface could be directly exposed to the electrolytes to suppress H2 evolution. With a set of carefully designed comparison study, they have identified that the oxidation of Cu2O into CuO by photo-generated holes is the main reason for the instability of Cu2O photocathode, although it has been long suggested that the instability is caused by the reduction of Cu2O into metallic Cu.
With Cu2O as the dark cathode and TiO2 as photoanode, their photocatalytic CO2 reduction system exhibits a stable performance over a 6-hour period, with an extremely high selectivity of 92.6% for carbonaceous products, whichis among the highest values ever reported.
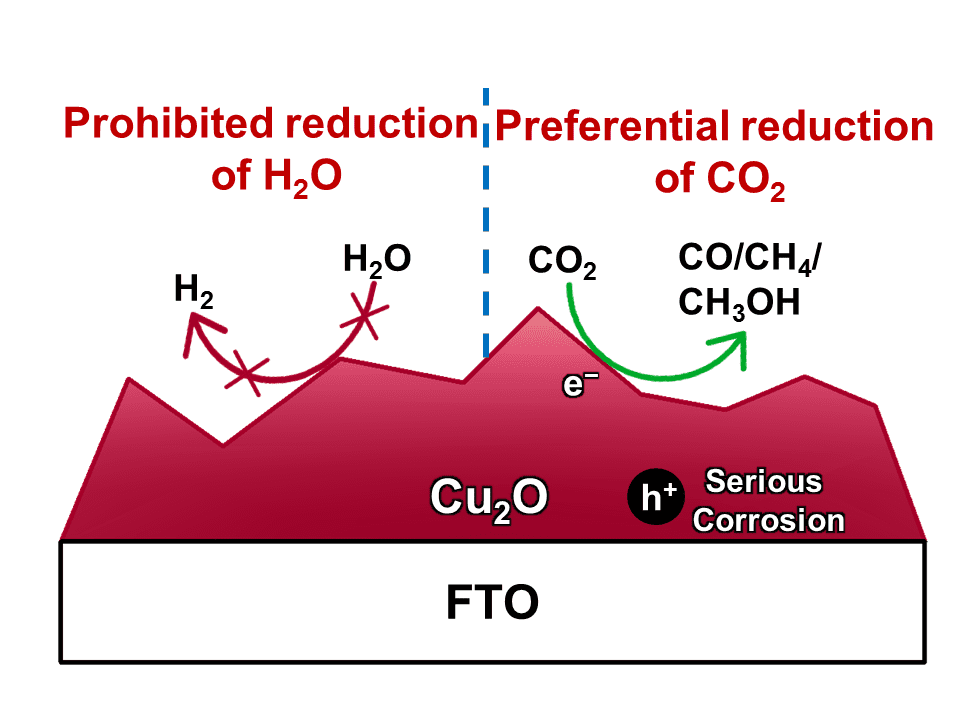
 The photocatalytic reduction of CO2 into chemicals and fuels is a promising way to address global warming and energy crisis. However, the hydrogen evolution reaction (HER) severely competes with CO2 reduction for electrons, leading to a low selectivity for carbonaceous products. To suppress H2 evolution, Cu2O has been applied as the cathode owing to its active sites for the preferential conversion of CO2 molecules. Unfortunately, Cu2O suffers from poor stability since its redox potentials lay within its band gap. A common strategy to improve the stability of Cu2O cathode is to deposit a protective layer (such as TiO2) onto Cu2O. But the protective layer will also prohibit the Cu2O surface from suppression the HER, which sacrifices the selectivity for carbonaceous products.
The photocatalytic reduction of CO2 into chemicals and fuels is a promising way to address global warming and energy crisis. However, the hydrogen evolution reaction (HER) severely competes with CO2 reduction for electrons, leading to a low selectivity for carbonaceous products. To suppress H2 evolution, Cu2O has been applied as the cathode owing to its active sites for the preferential conversion of CO2 molecules. Unfortunately, Cu2O suffers from poor stability since its redox potentials lay within its band gap. A common strategy to improve the stability of Cu2O cathode is to deposit a protective layer (such as TiO2) onto Cu2O. But the protective layer will also prohibit the Cu2O surface from suppression the HER, which sacrifices the selectivity for carbonaceous products.