Carbon dioxide is an abundant greenhouse gas. As such, a tremendous effort has been devoted to removing it from the atmosphere. One measure involves recycling carbon dioxide as a feedstock for chemical manufacturing, such as carbon dioxide hydrogenation, which can be used to produce useful hydrocarbons.
In their paper in Advanced Energy Materials, Dr. Zhaorong Huang from Cranfield University, and co-workers from Cranfield University and Queen Mary University of London, report the use of a thermoelectric material as a catalyst support and promotor for carbon dioxide hydrogenation. Using a specially designed reactor chamber, they achieve simultaneous thermoelectric energy harvesting and catalysis.
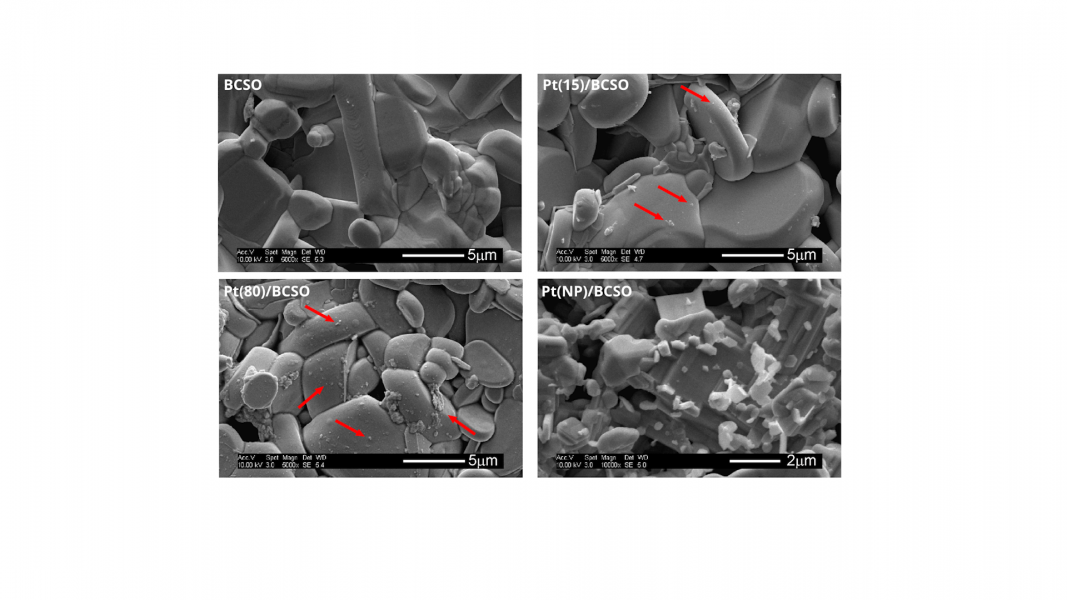
Platinum thin-film and nanoparticle catalysts based on a thermoelectric material, BSCO (BiCuSeO), were placed in a single-chamber reactor. The sample was heated from below, generating a large vertical temperature gradient across the sample under normal thermoelectric (TE) conditions, which was much less noticeable under reduced thermoelectric (RTE) conditions.
Conversion of carbon dioxide to carbon monoxide and methane was greatest under higher TE conditions, relative to RTE conditions. In the absence of Pt catalyst, BSCO was also found to be catalytically active.
Under TE conditions, different gas inlet compositions of carbon dioxide/hydrogen of 1:1 and 1:4 show that a higher hydrogen concentration leads to lower carbon monoxide selectivity and higher carbon dioxide conversion, but the Seebeck coefficient is not affected by the inlet gas composition.
For all samples, there is an excellent linear relationship between the logarithmic carbon dioxide hydrogenation conversion (ln(X)) and the ratio between the extra electrochemical energy induced by the TE effect and the thermal energy of an electron at the reaction surface (–eV/kbTh). This relationship shows that the Seebeck voltage (V) can control the thermal equilibrium conversion of the reaction, promoting conversion of carbon dioxide to carbon monoxide and methane.
To find out more about this thermoelectric material for carbon dioxide hydrogenation, please visit the Advanced Energy Materials homepage.