A team of University of Texas at Arlington chemists and engineers have proven that concentrated light, heat and high pressures can drive the one-step conversion of carbon dioxide and water directly into useable liquid hydrocarbon fuels.
This simple and inexpensive new sustainable fuels technology could potentially help limit global warming by removing carbon dioxide from the atmosphere to make fuel. The process also reverts oxygen back into the system as a byproduct of the reaction, with a clear positive environmental impact, researchers said.
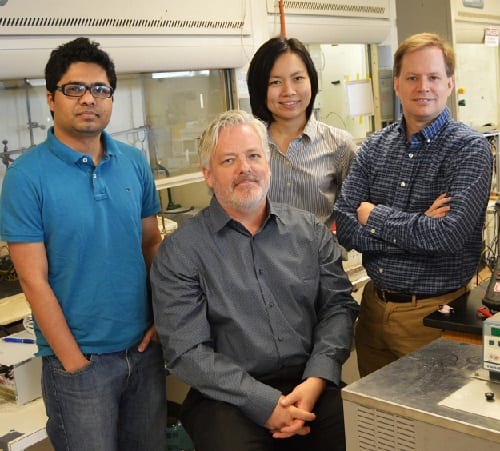
From left: Mohammad Fakrul Islam, Frederick MacDonnell, Wilaiwan Chanmanee and Brian Dennis. Source: UTA
“Our process also has an important advantage over battery or gaseous-hydrogen powered vehicle technologies as many of the hydrocarbon products from our reaction are exactly what we use in cars, trucks and planes, so there would be no need to change the current fuel distribution system,“ said Frederick MacDonnell, UTA interim chair of chemistry and biochemistry and co-principal investigator of the project.
The researchers demonstrate that the one-step conversion of carbon dioxide and water into liquid hydrocarbons and oxygen can be achieved in a photothermochemical flow reactor operating at 180 to 200°C and pressures up to 6 atmospheres.
“We are the first to use both light and heat to synthesize liquid hydrocarbons in a single stage reactor from carbon dioxide and water,” said Brian Dennis, UTA professor of mechanical and aerospace engineering and co-principal investigator of the project. “Concentrated light drives the photochemical reaction, which generates high-energy intermediates and heat to drive thermochemical carbon-chain-forming reactions, thus producing hydrocarbons in a single-step process.”
The hybrid photochemical and thermochemical catalyst used for the experiment was based on titanium dioxide, a white powder that cannot absorb the entire visible light spectrum.
“Our next step is to develop a photo-catalyst better matched to the solar spectrum,” MacDonnell said. “Then we could more effectively use the entire spectrum of incident light to work towards the overall goal of a sustainable solar liquid fuel.“
See the original publication, published in Proc. Natl. Acad. Sci. USA here.