 Clean and affordable energy storage and conversion technologies are required to solve one of the most pertinent challenges facing society: sustainability of the energy sector.
Clean and affordable energy storage and conversion technologies are required to solve one of the most pertinent challenges facing society: sustainability of the energy sector.
Fuel cells are such a technology, which employ a technique called electrocatalysis – the use of a catalyst material to enhance electrochemical reactions, making them viable at an industrial level. The current industry standard material for electrocatalysis is platinum on carbon (Pt/C). While carbon isn’t difficult to source, platinum—a famously good catalyst which is chemically inert to high temperatures, nontoxic in its pure form, and resistant to corrosion—is very expensive, being comparatively rare.
New nanostructured material designs are therefore required to transform the affordability and performance of fuel cells to reach levels that are practical for the increasing demand of future transportation, backup, and stationary power applications.
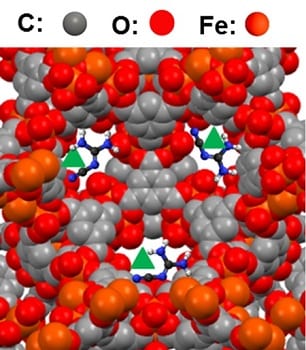
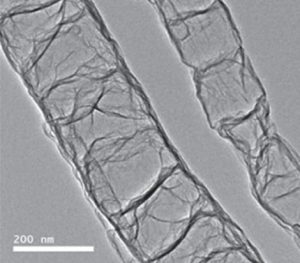
To accomplish this, collaborators from China, Korea, and the USA developed a new hybrid catalyst technology and applied it to the oxygen reduction reaction (ORR), a standard test for electrocatalyst materials. They took tubes of graphene doped with nitrogen impurities (N-GTs), which already have some degree of ORR activity, and reacted them with iron to create N-GT-rich iron–nitrogen–carbon catalysts.
Embedding Pt nanoparticles into this new matrix generated a novel hybrid Pt cathode with reduced platinum content compared to the Pt/C standard, but with improved ORR activity, stability, and fuel cell performance, all of which reduce the cost of the system while delivering better results.