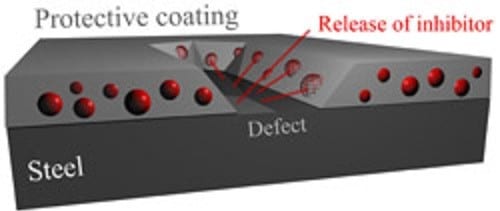
Research in corrosion protection has been increasing since the 18th century, especially with respect to steel. In the steel industry, corrosion protection is usually achieved by galvanizing with zinc, which dissolves instead of the iron when it comes into contact with corrosive media. However, during the production process, initial corrosive spots are formed right at the cut edge. An idea has been developed at the Max-Planck-Institut für Eisenforschung (MPIE) in Düsseldorf/ Germany to solve this problem – they suggest incorporating microcapsules that are filled with corrosion inhibitor, e.g. polyphosphomolybdate, into the zinc coating.
 As soon as the steel sheet is cut, the zinc starts to corrode and dissolve. This is the starting shot for the intelligent, second protective system: the capsules are released from the zinc onto the steel and smeared along the surface by the cutting device. The inhibitor can be released and thus protects the steel surface.
As soon as the steel sheet is cut, the zinc starts to corrode and dissolve. This is the starting shot for the intelligent, second protective system: the capsules are released from the zinc onto the steel and smeared along the surface by the cutting device. The inhibitor can be released and thus protects the steel surface.
“This is an intelligent protective system that automatically realises when and where corrosion happens, becomes active and stops again when the respective spot is healed”, explains Dr. Rohwerder, group leader in the Interface Chemistry and Surface Engineering department. “It works like a scratch in the skin: it is detected, healed and the initial status is restored.”
Three steps are required to prepare these smart coatings: loading of the silica microcapsules with the inhibitor, sealing them to avoid premature leaching, and finally incorporating the capsules into the zinc layer. The incorporation into the zinc layer is the most difficult part. Unmodified, the hydrophilic particles are repulsed by the zinc and only adsorb on the surface.
However, Tabrisur Rahman Khan, a PhD student from Bangladesh, has solved this problem. He has modified the particles with zinc affine functional groups, such as thiols, which make the solvation feasible.
Unfortunately, for efficient protection, a higher loading of the pores with the inhibitor must be realised. This is the focus of current research. Additionally, the concept of intelligent corrosion coatings has been expanded to systems with polymer coatings. Two Max-Planck and two Fraunhofer Institutes are sharing their competences with respect to nanocomposite coatings, agent containers, zinc coatings and the analysis of effective mechanisms in order to improve the protective coatings. “It is a huge challenge, but present results look very promising”, states Rohwerder.