Subtle changes in a molecule’s structure have important consequences for how a drug behaves. Polymorphism, the phenomenon where a compound exists in different crystal forms, in particular has long posed challenges.
“Polymorph screening has played a crucial role in pharma since different polymorphs of the same molecule can have different properties,” explained Emma Danelius, a postdoctoral fellow at UCLA School of Medicine. “A notorious case […] is that of the anti-HIV drug ritonavir. Originally, ritonavir was formulated based on the only known crystal form at that time. However, another crystal form with reduced solubility and bioavailability was discovered, [which] made the drug less effective for patients, and led to temporary market withdrawal.”
Danelius is part of a team of researchers led by Tamir Gonen, professor at UCLA School of Medicine, interested in developing a better way to identify polymorphs in order to better understand and identify structural nuances that could lead to more effective and safer pharmaceuticals.
In their recent study published in Advanced Biology, the scientists set their sights on paritaprevir, an oral macrocyclic drug used to treat chronic hepatitis C, whose varying crystal structures have eluded scientists for years despite its widespread clinical use.
Challenges studying macrocycles
X-ray diffraction is commonly employed to elucidate the three-dimensional structures of large molecules such as macrocycles — cyclic molecules characterized by ring sizes consisting of no less than 12 non-hydrogen atoms. Due to their significant size, macrocycles exhibit conformational complexity and flexibility, enabling them to adopt multiple conformations and interact with challenging protein targets. However, this flexibility also complicates the preparation of samples needed for X-ray diffraction.
“Traditionally, the substance needs to be crystallized, and the structure is determined by analyzing the patterns obtained from X-ray diffraction of the well-ordered crystal,” explained Danelius. Crystallization procedures can be time-consuming and, depending on the molecule, challenging to achieve.
“Paritaprevir […] falls within a category of large and flexible pharmaceuticals, which are notoriously difficult to crystallize and study using X-ray techniques,” she added. “We needed new ways to study them experimentally.”
Paritaprevir helps manage hepatitis C infections by binding to one of the virus’ key enzymes called non-structural 3/4A (NS3/4A) protease, which is vital to viral replication and assembly. “Without NS3/4A protease, the hepatitis C virus cannot be replicated and assembled into mature viral particles,” explained Guanhong Bu, a postdoctoral fellow at UCLA School of Medicine and another of the study’s contributing authors.
Macrocyclic drugs, such as paritaprevir, are susceptible to polymorphism owing to their size and flexibility. However, despite this propensity, only two crystal polymorphs have been identified using powder or single crystal X-ray diffraction methods. There is also a notable absence of structures in public databases depicting paritaprevir bound to NS3/4A protease.
Electron diffraction gets new life
In response to this scarcity of structural data, the team implemented an approach they had developed back in 2013, known as microcrystal electron diffraction, or MicroED. This innovative technique allows for the determination of three-dimensional structures using electron diffraction from minuscule crystals ranging from micron to sub-micron in size — as small as a billionth the size as those required for single crystal X-ray diffraction.
“For decades it was accepted that electron diffraction was obsolete,” said Gonen. “We figured out how to perform it in an efficient and powerful way. A decade later, we are still finding important samples that remained recalcitrant to other methods but which are ideal for MicroED. Macrocyclic drugs, like paritaprevir, is one such example.”
“In solution, flexible molecules, such as paritaprevir, exist as an ensemble of rapidly interconverting conformations,” added Bu. “When we study these molecules in the solid state, we don’t get the full picture, but when using microcrystals and MicroED, it seems like we can catch some of the [important] conformational dynamics.”
The team successfully identified two polymorphs of paritaprevir, designated as α and β, with one of them being previously unreported. “We noticed when comparing the polymorph structures that their conformation — how they fold in 3D — are different,” said Danelius.
“When we used both conformations in computer-aided molecular docking to the target hepatitis C virus NS3/4A protease, only one conformation [β] could be successfully docked into the active site — the key position for the protease activity and the predicted site for drug binding,” she continued.
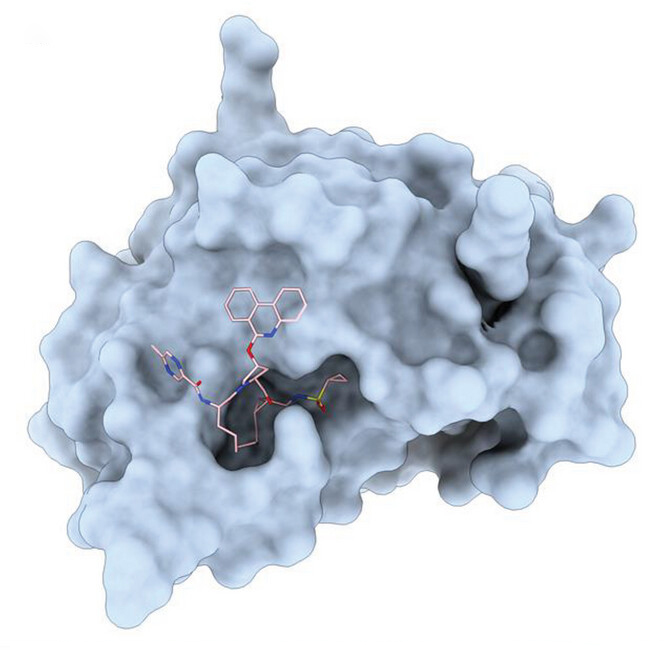
This analysis allowed the team to investigate how the antiviral drug potentially binds to and inhibits protease activity using computational simulations. This, say the team, underscores the importance of experimentally determining the correct conformation for precise target docking, especially for this class of molecules.
Furthermore, the computer-aided molecular docking simulations revealed that the macrocyclic core of paritaprevir β remains almost the same both in the crystals and when bound within the enzyme, while the molecule’s additional substituents adjusted to fit the active site. “This suggests that […] having the right pre-organized macrocyclic core conformation by experimental method could lead to better computational simulation results for this class of molecules,” said Bu.
This could entail focusing solely on isolating the β conformation for medicinal applications, leveraging its superior binding affinity. Additionally, these findings serve as a crucial foundation for enhancing the binding capability of paritaprevir and similar inhibitors to the hepatitis C virus NS3/4A serine protease.
The team writes that this approach could transcend conventional methodologies, and add an important tool for researchers to refine drug candidates based on specific structural insights, potentially optimizing treatment efficacy in a more meaningful way.
“What we are seeing is an emergence of MicroED as an important tool to guide drug formulation,” said Gonen. “In the future, I predict that the FDA would require MicroED structures of drugs before giving their approvals.”
Reference: Tamir Gonen, et al., Polymorphic Structure Determination of the Macrocyclic Drug Paritaprevir by MicroED, Advanced Biology (2024). DOI: 10.1002/adbi.202300570