Actinides (such as uranium, thorium, and plutonium) and their alloys occupy a well-known important position in nuclear science and technology. With the depletion of the traditional energy industries such as global oil, natural gas, and coal, it is estimated that by the middle of this century, nuclear power will occupy a large proportion of human energy consumption. Therefore, the scientific research on actinide in the past ten years has been the continuous concern of the international scientific community.
Currently, a long-standing debate on systems containing the actinide element is the extent of localization and participation of the 5f orbitals in chemical bonding across the actinide series.
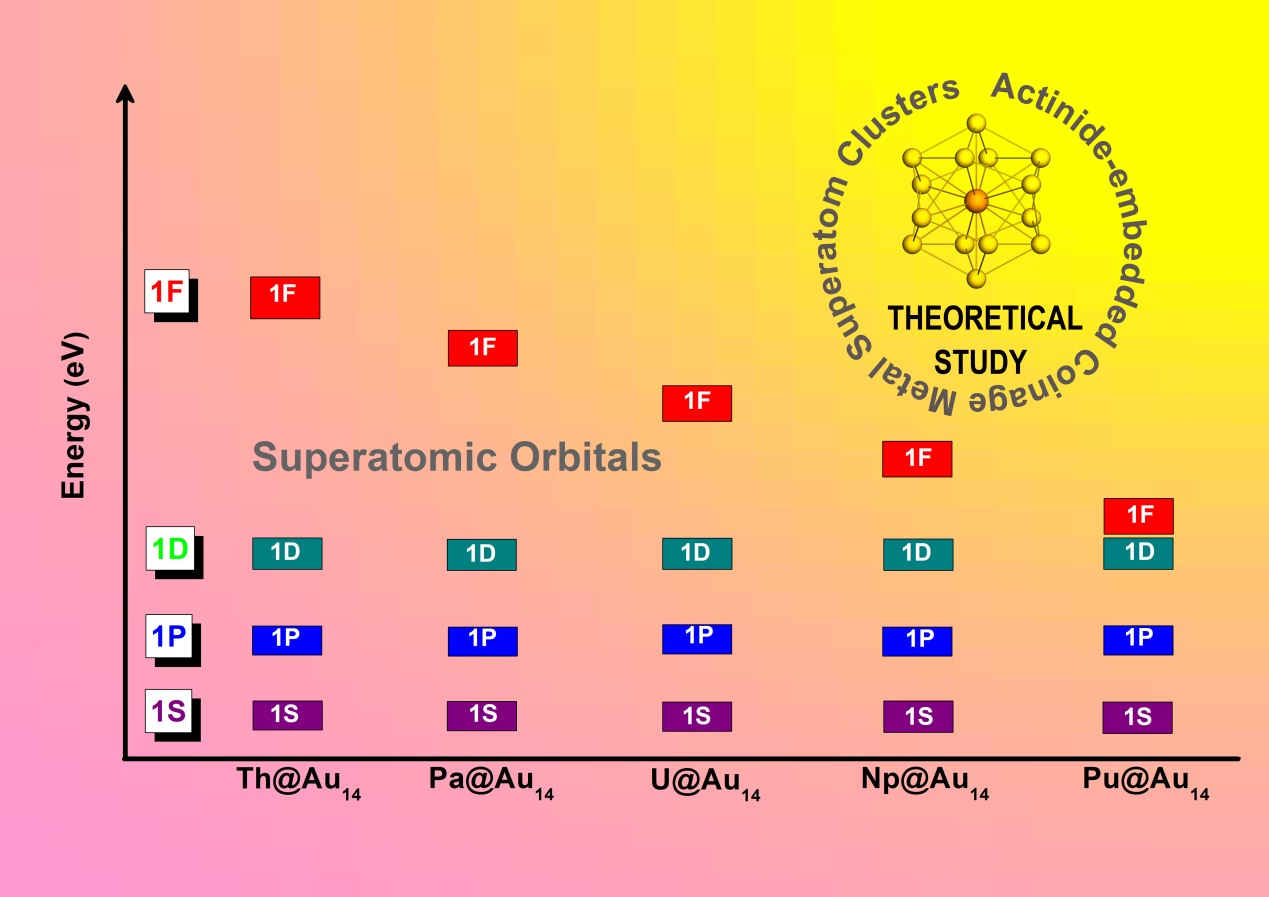
In their paper published in Advanced Theory and Simulations, Zhigang Wang and his colleagues at the Institute of Atomic and Molecular Physics, Jilin University designed a series of light-actinide-embedded coinage metal superatomic models and revealed that the 5f orbitals have dual nature in superatomic bonding for protactinium, uranium, neptunium, and plutonium.
More specifically, they found that the partial 5f electrons are active and could be preferentially excited to 6d shells to satisfy Jelliumic chemical bonding of the 18-electron rule. In contrast, the remaining 5f electrons are more localized and present anti-ferromagnetic or ferromagnetic couplings for the spin arrangements between actinides and confined metal out-shell, and largely spin density localized at the actinide atom.
This work not only offers a new recipe for breeding magnetic superatoms but also paves the way for designing new superconducting materials and heavy-fermion systems.
Kindly provided by the Authors.