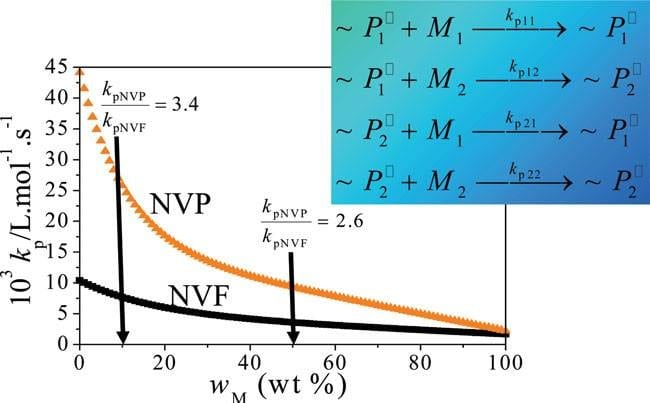
Over the past few years, application of specialized pulsed-laser techniques has led to the finding that the rate coefficient for chain growth of water-soluble monomers varies with the ratio of monomer to water in solution. Accounting for this effect has led to new understanding of how polymerization rate and polymer molar masses are affected by reaction conditions for homopolymerization of monomers such as methacrylic acid, N-vinylpyrrolidone (NVP) and N-vinylformamide (NVF), used to produce polymers for wastewater treatment, pharmaceutical and consumer personal care markets.
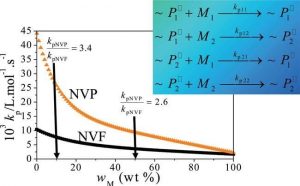 A collaboration between the groups of Robin Hutchinson (Queen’s University, Kingston, Ontario) and Igor Lacík (Slovak Academy of Science, Bratislava) has extended this understanding to aqueous-phase copolymerization. The influence of initial monomer concentration and comonomer composition on reaction rate, polymer molar masses and copolymer composition was studied for the aqueous-phase copolymerization of NVP and NVF. A simple model assuming terminal copolymerization kinetics captures the kinetic and molar mass behavior of this system, using the same rate coefficients determined from the homopolymerization studies of NVP and NVF. Thus, knowledge of the propagation kinetics is shown to be the key factor for the rational design of new products by aqueous-phase copolymerization.
A collaboration between the groups of Robin Hutchinson (Queen’s University, Kingston, Ontario) and Igor Lacík (Slovak Academy of Science, Bratislava) has extended this understanding to aqueous-phase copolymerization. The influence of initial monomer concentration and comonomer composition on reaction rate, polymer molar masses and copolymer composition was studied for the aqueous-phase copolymerization of NVP and NVF. A simple model assuming terminal copolymerization kinetics captures the kinetic and molar mass behavior of this system, using the same rate coefficients determined from the homopolymerization studies of NVP and NVF. Thus, knowledge of the propagation kinetics is shown to be the key factor for the rational design of new products by aqueous-phase copolymerization.