The catalytic reduction of N2 to NH3 in the Haber–Bosch process is a fundamental pillar of today’s chemical industry. The process can occur over a metallic Fe-based catalyst under extreme reaction conditions. Conversely, in nature, the enzyme nitrogenase can reduce N2 to NH3 at ambient temperature and pressure, prompting the development of catalyst systems that can mimic nitrogenase and achieve a similar energy efficient transformation of N2 to NH3.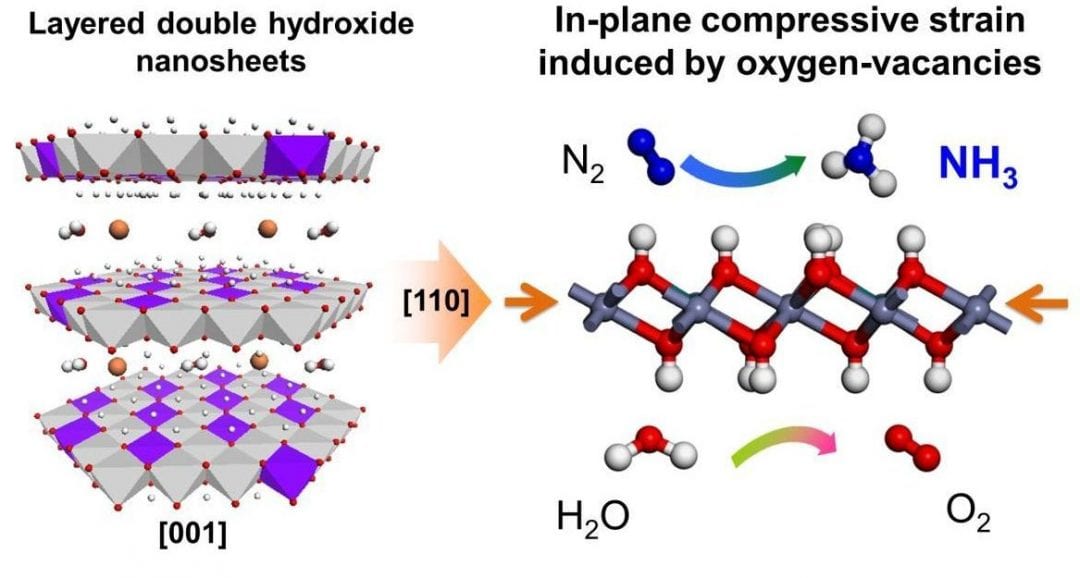
Semiconductor photocatalysts containing an abundance of active coordinatively unsaturated metal sites may be able to activate N2 under photoexcitation. However, most semiconductor photocatalysts reported to date exhibit weak visible light absorption ability around 420–500 nm, severely handicapping their solar spectrum-utilization efficiency for N2 fixation. There exists a pressing need and also a strong fundamental scientific drive to develop new photocatalysts with a wide-range photoresponse for the photoreduction of N2.
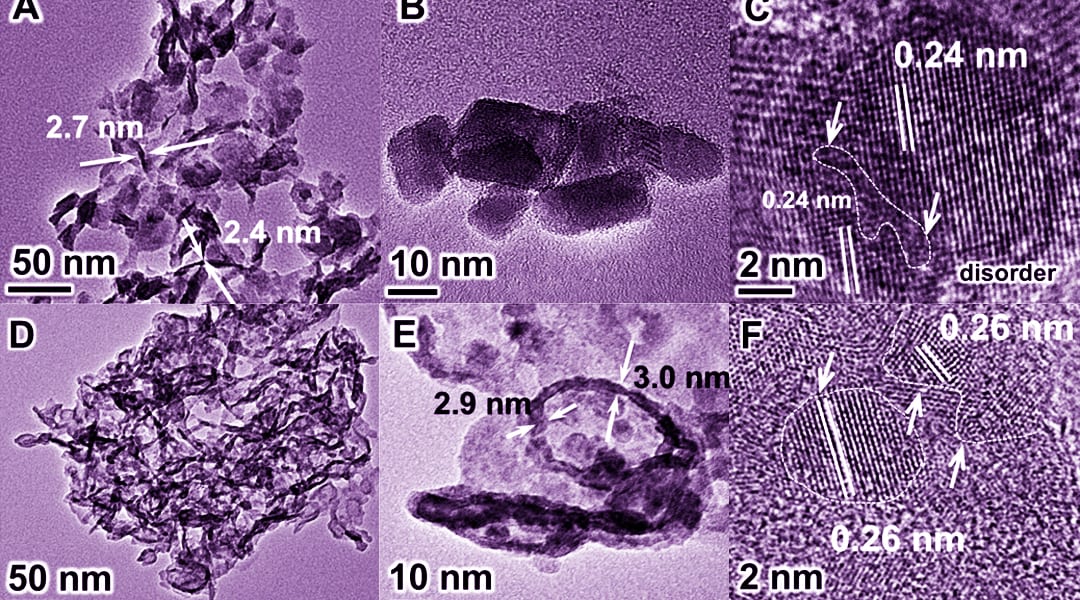
Tierui Zhang, Technical Institute of Physics and Chemistry (Chinese Academy of Sciences), Geoffrey I.N. Waterhouse, The University of Auckland, Dermot O’Hare, University of Oxford and their teams, for the first time, facilely synthesized a new class of metal-to-metal charge-transfer (MMCT) systems. MIIMIII-layered double hydroxide (LDH) (MII = Mg, Zn, Ni, Cu; MIII = Al, Cr) nanosheet photocatalysts with the efficient photoreduction of N2 (especially the CuCr-LDH nanosheets, which showed a quantum yield ≈0.10 % at 500 nm).
Detailed characterizations reveal that the LDH nanosheet structure imparted structural distortions and compressive strain, which enhanced N2 adsorption and aided electron transfer from the LDH photocatalyst to N2 under wide-range photoirradiation, thereby promoting NH3 evolution.
This work demonstrates an extremely promising new pathway for the reduction of N2 to NH3 under visible light at ambient temperature and pressure by using LDH based visible-light responsive catalysts.
The research was recently published in Advanced Materials.