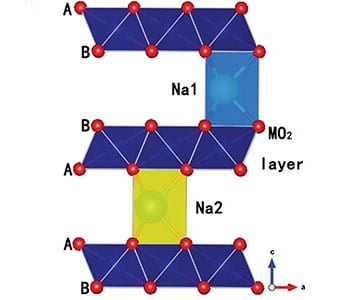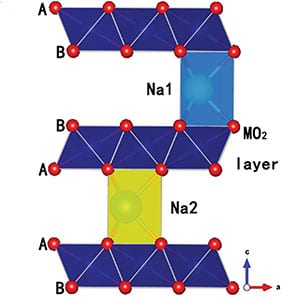
Schematic illustration of the P2-type structure.
Chinese researchers recently proposed a positive Na-ion electrode based on a P2 layered oxide with copper. An air-stable Na7/9Cu2/9Fe1/9Mn2/3O2 was synthesized following a conventional solid state reaction. The addition of iron reduced the overall cost of the electrode, moreover improving the structural stability and increasing the sodium content. A higher sodium content was found to improve both the capacity and the storage voltage.
Developing batteries with long life cycles and a high energy conversion efficiency is paramount for implementing large-scale portable energy storage. Lithium ion batteries are an established power source, which have been widely used in small electronic devices and are now a promising candidate for use in electronic vehicles. The future of their use in such large-scale applications, however, may be restricted due to limited resources and high production costs. With abundant and inexpensive resources, sodium-ion batteries have recently garnered attention as a potential alternative to their Li-ion contenders. While several negative electrode materials for Na-ion batteries have been determined, developing an appropriate positive electrode structure remains an important route for improvement. The most promising to date are those based on a P2 layered oxide structure. Encouraging results have been found for Na electrodes incorporating nickel or cobalt, however these metals may be unsuitable for large-scale storage systems due to their potential expense.
The researchers demonstrate the electrochemical performance of their copper-based positive electrode, which has a particularly attractive cycling stability (85% capacity retention after 150 cycles at 1C rate without phase transformation). A high Coulombic efficiency and high average operating voltage further increase the attractiveness of this non-toxic, inexpensive fuel cell component.
The rate performance could be further improved by additional transition metal doping or by decreasing the particle size. The electrode structure is tested in a full battery setup with a hard carbon negative electrode. The resulting energy conversion efficiency and capacity retention indicate that the high sodium content, air-stable positive electrode is a promising candidate for future, environmentally friendly, large-scale energy applications.
Advanced Science is a new journal from the team behind Advanced Materials, Advanced Functional Materials, and Small. The journal is fully Open Access and is free to read now at www.advancedscience.com.