Image credit: Lukasz Szmigiel on Unsplash
It is time to get serious about nitrous oxide (N2O) as it three hundred times more potent than carbon dioxide (CO2) and possessing an estimated atmospheric lifetime of 114 years, the gas accounts for 7% the US’s CO2 equivalent greenhouse gas emissions and has risen to become the world’s most notorious ozone depleting agent. Despite its consequences, insufficient effort has been made to minimize the compound’s emission. With these effects, it is imperative that we recognize that the issue of N2O as not a laughing matter.
What are the main sources of N2O?
Agricultural soil management is responsible for about 75% of N2O emissions. The remainder is derived from wastewater treatment (6%), stationary combustion (5%), industry and chemical production (5%), manure management (4%), transportation (4%), and other sources (4%). Up until 1920, the concentration of N2O in the atmosphere for the past 800,000 years remained stable at 280 ppb. Since this time, N2O levels have been measured at record-setting levels of 331 ppb in 2019, primarily attributed to the heavy and increasing use of nitrogen fertilizer.
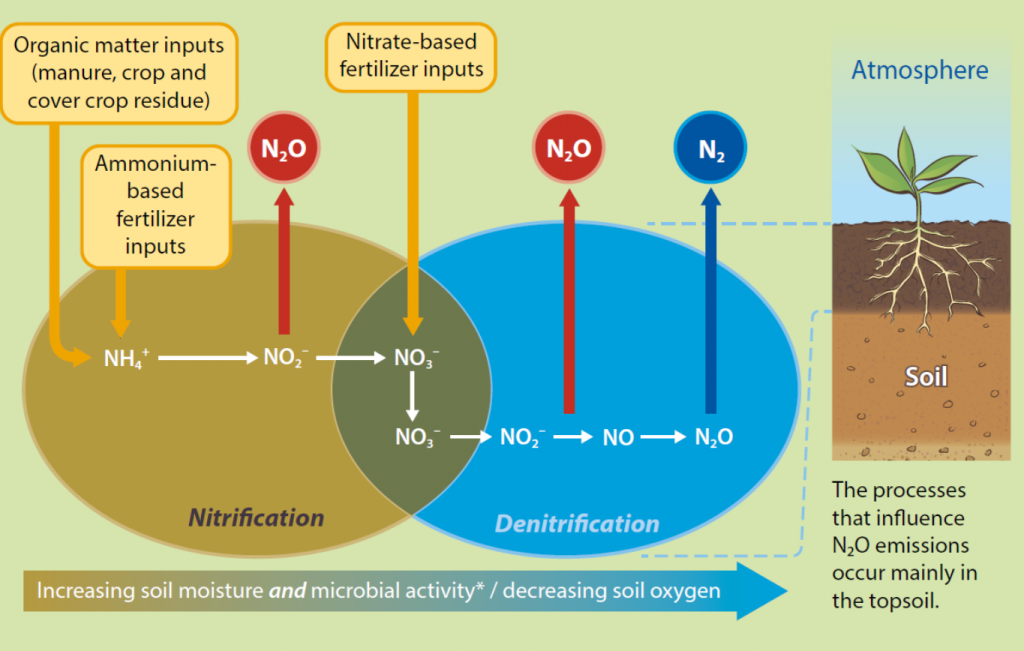
While the use of ammonia-sourced nitrogen fertilizer has become necessary to support the crop yields required to sustain the growing global population, any unused fertilizer applied to the soil beyond the needs of the plant is transformed by microbes in a succession of reactions, as illustrated in Figure 1.
How do we prevent the formation of fertilizer derived N2O?
As a materials chemist, I immediately turn to catalysts for my solutions. Rather than devising costly methods to micromanage fertilizer concentrations in fields, why not devise a light-driven catalyst capable of stopping nitrous oxide formation in the first place. Using energy from the sun, the initial step leading to nitrous oxide formation, or the reaction converting NO3– back to NH4+, could be reversed, allowing excess ammonium to stay in fields to be eventually utilized by crops. If made biocompatible, such a system could be spread into soil with the fertilizer and enable the following reaction equation 1 when exposed to sunlight.
HNO3 + 2H2O + hv –> NH4OH + 2O2
Alternatively, a biological method could be used. Much like how bacteria have been used in sewage treatment plants to generate fertilizer, specialized microbiota can be designed to reverse nitrous oxide formation. However, this method can result in devastating environmental effects. Short generational times and genetic exchange across strains means there is no telling how such a designed bacterium would act in the field. As well, extensive biological compatibility testing would need to be conducted to see how the bacteria interacts with flora and fauna.
Controlling N2O emissions is an urgent global problem. If nothing is done, we may find ourselves the casualties of our own ingenuity. But this is not to say that the solution will come easily. The challenge with the proposed design would be to engineer a photocatalyst that can be mixed and spread with fertilizer, capture light, without interfering with the complex soil makeup crucial to plant growth.
With this vision in mind, let us work together to make this technology a reality so those after us may share a laugh on this earth another day.
Written by: Geoff Ozin and Jessica Ye
References: Elizabeth Verhoeven, et al., N2O emissions from California farmlands, California Agriculture, 2017, 71(3), DOI 10.3733/ca.2017a0026.