Photocatalytic water splitting has long been viewed as an important future technology for solar energy conversion and the sustainable delivery of H2 as an energy carrier. 2D graphitic carbon nitride (g-C3N4) has recently emerged as a promising new semiconductor photocatalyst for renewable energy applications including water splitting on account of its excellent photocatalytic activity, non-toxicity and low cost. However, the large band gap (≈2.7 eV) of pristine g-C3N4 hinders its ability to fully utilize the solar spectrum.
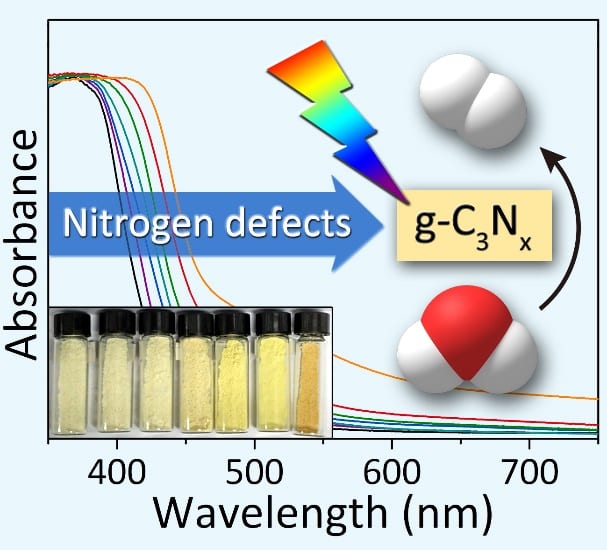
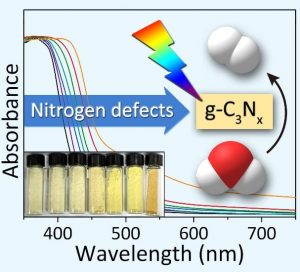 Introducing nitrogen defects has been previously shown to narrow the bandgap and improve the solar harvesting properties of g-C3N4. However, many of the synthetic procedures reported to date for introducing nitrogen defects into g-C3N4 demand harsh experimental conditions and afford minimal control over the type and abundance of nitrogen defects, often resulting in g-C3N4 photocatalysts with poor performance and severe electron-hole pair recombination rates under visible light. Simple and reliable experimental methods are needed for the controlled introduction of nitrogen defects into g-C3N4.
Introducing nitrogen defects has been previously shown to narrow the bandgap and improve the solar harvesting properties of g-C3N4. However, many of the synthetic procedures reported to date for introducing nitrogen defects into g-C3N4 demand harsh experimental conditions and afford minimal control over the type and abundance of nitrogen defects, often resulting in g-C3N4 photocatalysts with poor performance and severe electron-hole pair recombination rates under visible light. Simple and reliable experimental methods are needed for the controlled introduction of nitrogen defects into g-C3N4.
Recently, Tierui Zhang and co-workers at Technical Institute of Physics and Chemistry of the Chinese Academy of Sciences reported a novel alkali-assisted strategy for the synthesis of nitrogen deficient g-C3N4. The strategy involved mixing g-C3N4 precursors (melamine, urea or thiourea) with small amounts of an alkali (KOH, NaOH, or Ba(OH)2), followed by calcination of the resulting mixtures at 550 oC in an alumina crucible. By simply increasing the alkali:organic precursor weight ratio in the starting mixture, more nitrogen defects could be introduced into the 2D g-C3N4 sheets, progressively reducing the bandgap from 2.68 to 2.36 eV.
Deeper investigations using NMR and DFT calculations pinpointed the types of defects formed and their contribution to the band structure. It was concluded that nitrogen deficiencies generated in-situ by the presence of the alkali lowered the energy of the g-C3N4 conduction band, thereby allowing increased visible light absorption whilst maintaining good charge separation efficiency. Accordingly, the nitrogen-deficient g-C3N4 photocatalysts demonstrated superior hydrogen evolution performance compared to pristine g-C3N4 under visible excitation.
The study describes a very simple and general method for introducing nitrogen defects into the 2D structure of g-C3N4, allowing precise control over the abundance and types of defect. The findings provide valuable new knowledge about the synthesis of g-C3N4 and also support wider research efforts examining the critical roles of defects in enhancing light absorption and charge transfer processes in 2D semiconductor photocatalysts