 Polyurethanes are interesting materials for biomedical application because of their low toxicity, excellent biocompatibility, potential biodegradability, and low thrombogenicity. For potential use as controlled drug delivery systems the polymer has to be combined with distinct biological functions. The controlled release of therapeutic molecules can be achieved by anchoring the active agent to polymer structures by means of physical interactions or by covalent linkages. Hence, the functionalization of polyurethane materials is a topic of great interest.
Polyurethanes are interesting materials for biomedical application because of their low toxicity, excellent biocompatibility, potential biodegradability, and low thrombogenicity. For potential use as controlled drug delivery systems the polymer has to be combined with distinct biological functions. The controlled release of therapeutic molecules can be achieved by anchoring the active agent to polymer structures by means of physical interactions or by covalent linkages. Hence, the functionalization of polyurethane materials is a topic of great interest.
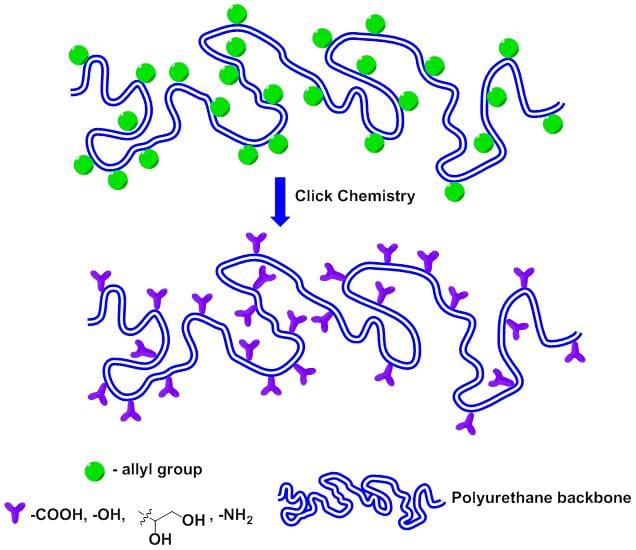
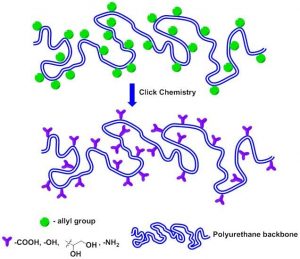
The incorporation of carbohydrate moieties in polycondensation and polyaddition polymers, such as polyamides, polyesters, and polyurethanes is an interesting approach to yield novel sustainable and biodegradable materials. However, linear polymerization of monomers derived from carbohydrates is not straightforward. Carbohydrate-based compounds usually possess an excess of functional groups that upon polycondensation would lead to undesirable cross-linking reactions unless special precautions are taken.
Juan A. Galbis and co-workers (University of Seville, Spain) have prepared new aromatic and aliphatic multifunctional polyurethanes by applying the thiol-ene reaction to L-arabinitol-based polyurethanes with multiple pendant O-allyl groups. This technique allows the on-demand functionalization of PU materials without altering the polymer backbone. Thus, the introduction of 1,2-dihydroxyl, carboxyl, and amine groups in the polymer structure has been successfully achieved. This strategy provides a simple and versatile platform for the design of new materials whose functionality can be used to anchor diverse drugs, proteins or other relevant biologically active molecules.