Immunological tolerance is important with regards to several physiological settings. First of all, tolerance to self-molecules ensures that our immune cells do not attack our own body’s peptides – a process that is disturbed in autoimmune and autoinflammatory diseases. Immunological tolerance to ingested, inhaled or topically applied molecules plays a role in atopy and allergic diseases. Tolerance to microbes is vital for the existence of the gut microbiome and other commensals. And finally, maternal tolerance to ‘altered self’ ensures that a fetus – who is half paternally derived – is not rejected by the mother.
In their ‘Think again’ article published in BioEssays, Megan Barnet and colleagues discuss these latter two examples of immune tolerance with a focus on how they can provide insights into cancer tolerance. Cancer cells are derived from self, but – via mutations – malignant clones have acquired distinguishing non-self features. The authors argue that cancer cells utilize many of the same pathways seen in physiological tolerance to escape immunological detection.
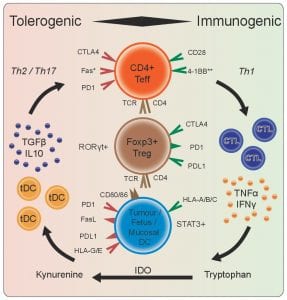
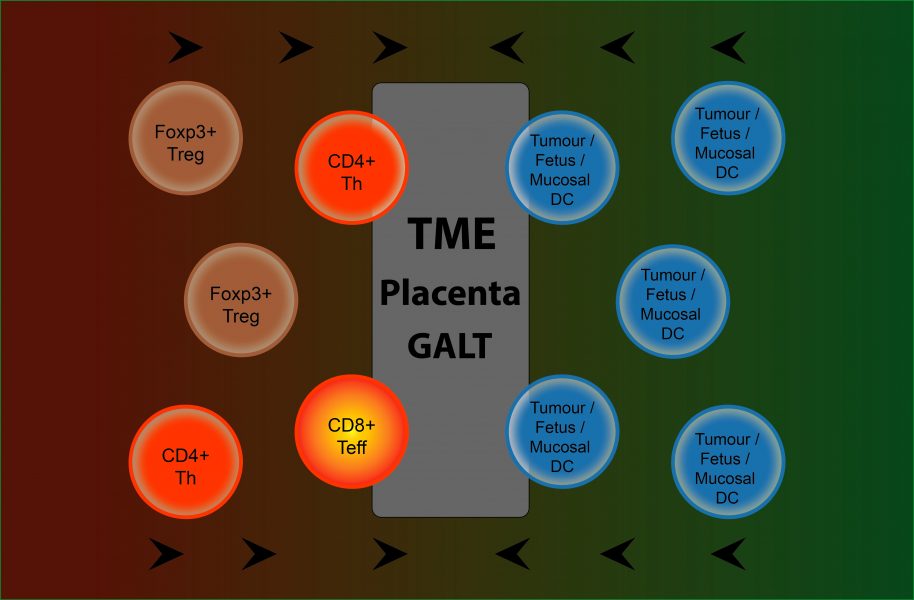
Shared tolerance mechanisms between the gut-associated lymphoid tissue (GALT) and the tumor microenvironment (TME), for example, include high expression of CTLA4 (cytotoxic T-lymphocyte-associated protein 4). CTLA4 is a protein receptor that functions as an immune checkpoint and downregulates immune responses.
The placental interface also shares tolerance mechanisms with the tumor microenvironment. PDL1 (programmed death ligand 1) is a transmembrane protein that acts as a negative feedback molecule to limit immune stimulation. It is constitutively expressed in (fetal) trophoblasts and incrementally increases from first- to third-trimester human placental tissue. With regards to cancer, targeting PDL1 with monoclonal antibodies is an established treatment in many types of solid cancers. Another example is the major histocompatibility complex (MHC). Fetal trophoblasts do not express class II MHC molecules and have restricted classical class I expression. An alteration of MHC expression patterns is also a strategy employed by both pathogens and cancer cells to achieve immune evasion.
Overall, a better insight into the mechanisms of physiological immune tolerance may therefore ultimately lead to new therapeutic opportunities to treat cancer.