Image credit: Jake Gard on Unsplash
A recent report described an innovative rechargeable metal-nitrogen battery based on a graphene-supported palladium (Pd) cathode and a polished aluminum (Al) anode interfaced with an ionic liquid electrolyte comprised of aluminum chloride/1-butyl-3-methylimidazolium chloride.
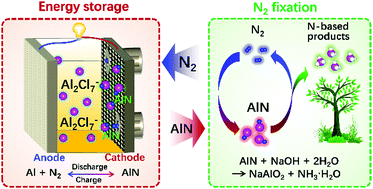
Remarkably, the Al-N2 battery was shown to serve the dual purpose of not only storing and retrieving energy, but also being able to fix the nitrogen (N2) stream as ammonia (NH3).
To amplify, the working principles of this novel Al-N2 battery are shown in the figure, and the balanced electrochemical reactions operating in the battery are presented below:
Anode: 2Al + 14AlCl4– ↔ 8Al2Cl7– + 6e–
Cathode: 8Al2Cl7– + N2 + 6e– ↔ 2AlN + 14AlCl4–
Overall: 2Al + N2 ↔ 2AlN
The Al-N2 battery demonstrated a faradaic efficiency of 51.2%, beyond the 5% of earlier reported M-N2 batteries based on lithium (Li) and sodium (Na) alkali metal anodes.
In comparing the Al-N2 to the prior art Li-N2 battery, it is noteworthy that the free energy of formation of ΔGf(AlN) = -287 kJ/mol is more negative than for ΔGf(Li3N) = -154 kJ/mol. Thermodynamically, this indicates the spontaneity of the reaction Al(s) + N2(g) –> 2AlN(s) is greater than 6Li(s) + N2(g) –> 2Li3N(s), and the open circuit voltage for Al-N2 (0.99 V) proves to be larger than Li-N2 (0.53 V). Furthermore, the standard reduction potential of Al being less than Li provides greater chemical stability for Al compared to Li. In addition, the natural Al2O3 passivation layer on Al confers upon it greater safety in its handling compared to Li.
Aside from the ability of Al-N2 to function as a rechargeable battery, it “kills two birds with one stone” by also functioning as a N2 fixation device by producing an aqueous NH3 solution from the electrochemically generated AlN according to the balanced alkali-base dissolution equation:
AlN + NaOH + 2H2O –> NaAlO2 + NH3•H2O
The N2 fixation performance of the Al-N2 battery is impressive, enabling an NH3 production rate of 27.1 mg/gcat.h.
Overall, the invention of the dual purpose energy storage-nitrogen fixation Al-N2 device speaks well for a distributed and sustainable future form of agriculture — a vision which portends an interesting eco-friendly alternative to the energy and greenhouse gas intensive Haber–Bosch process, and emerging green ammonia aqueous electrochemical N2 reduction alternative technologies.
Written by: Geoffrey Ozin
Solar Fuels Group, University of Toronto Ontario, Canada, Email: [email protected], Web sites: www.nanowizard.info, www.solarfuels.utoronto.ca, www.artnanoinnovations.com.
References:
Guo, Ying, et al., A Rechargeable Al-N2 Battery for Energy Storage and Highly Efficient N2 Fixation, Energy & Environmental Science (2020). DOI: 10.1039/D0EE01241F
Wang, Lu, et al., Greening ammonia toward the solar ammonia refinery, Joule 2.6 (2018). DOI: 10.1016/j.joule.2018.04.017

















