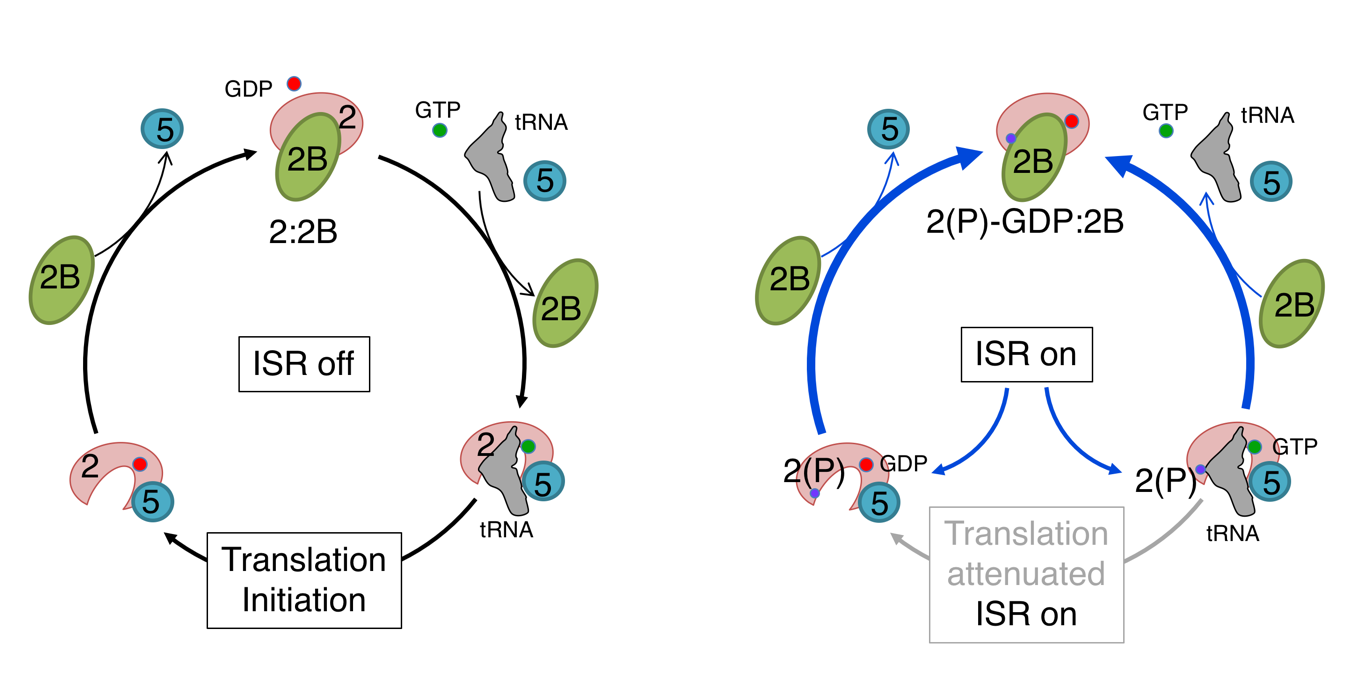
The integrated stress response (ISR) is a highly-conserved pathway used by cells to respond to diverse cellular stresses. Cells use a variety of sensors to perceive changing conditions and must respond in a timely and appropriate fashion so that balance and normal cellular activities can resume. Over the last fifteen years, the ISR has emerged as a critical and highly-conserved regulatory mechanism responsible for reprogramming gene expression at multiple levels. Central to this molecular response is a complex and not fully understood mechanism used to regulate how and which proteins are made by ribosomes in eukaryotes. Cells unable to respond appropriately or that fail overcome the perceived stimulus for an extended period or regulate the ISR in a timely fashion often leads to death or aberrant behavior. In humans this can contribute to a range of disorders. A new review, published in WIREs RNA, summarises recent studies that have shed light into how the translational control mechanism at the center of this regulatory pathway works.
In this recent review, author of the paper, Graham Pavitt, provides an overview of the ISR and then focuses on the action and control of key protein synthesis factors that are central to the mechanism, highlighting recent research that has contributed to our improved understanding of the translational control elements that are at the heart of this response. The action of three key protein synthesis factors eIF2, eIF2B and eIF5 is described in detail. eIF2 is critical for delivering initiator tRNA to ribosomes and accurately finding protein synthesis start codons on almost all mRNAs, while eIF2B and eIF5 take it in turns to interact with eIF2 and regulate its activity, but how does each protein actually work and what happens when the ISR is switched on by phosphorylation of eIF2?
Although it is well established that the outcome of the ISR is translation attenuation and enhanced selective translation of specific mRNAs, new insights in this paper include the observation that some protein complexes containing eIF2 and eIF5 can be disturbed by eIF2B to ensure tight regulatory control, enabling a new model to be proposed to account for how these factors each contribute to a translational control hub within the ISR. Downstream of these control events, specific ISR genes become activated and recent studies have improved our understanding of more diverse mechanisms of gene-specific controls.
Several diseases where elevated ISR responses occur are described in a WIREs article, as well as studies that have identified and characterized compounds that alter ISR response including kinase and phosphatase inhibitors as well as agonists that stimulate eIF2B function. Finally, this articles shows that there are an increasing number of studies using mouse models of diverse complex human conditions which point to future intervention strategies where using compounds to modulate the ISR may be of therapeutic value.
Kindly contributed by the Author.