A myriad of optoelectronic devices, such as photovoltaics or field-effect transistors, are based on p-conjugated polymers. The usual synthetic route follows reaction schemes including Suzuki–Miyaura and Migita–Kosugi–Stille cross-coupling reaction. A promising alternative is based on the direct arylation polycondensation reaction that enables elimination of several reaction steps. One drawback, however, is the associated formation of partially insoluble cross-linked structures in the polymer chains resulting from non-selective C-H activation.
A myriad of optoelectronic devices, such as photovoltaics or field-effect transistors, are based on p-conjugated polymers. The usual synthetic route follows reaction schemes including Suzuki–Miyaura and Migita–Kosugi–Stille cross-coupling reaction. A promising alternative is based on the direct arylation polycondensation reaction that enables elimination of several reaction steps. One drawback, however, is the associated formation of partially insoluble cross-linked structures in the polymer chains resulting from non-selective C-H activation.
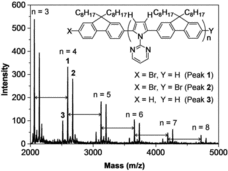
One approach to tackle this issue is the introduction of site selectivity into the polycondensation reaction. Takaki Kanbara and co-authors focus on the Ru-catalyzed direct arylation of pyrrole derivatives bearing a directing group. This results in cross coupling reaction at the a-position of the pyrrole monomer without b-position protection. In particular, they successfully proof the directed site-selective polycondensation of 1-(2-pyrimidinyl)pyrrole with 2,7-dibromo-9,9-dioctylfluorene with the 2-pyrimidinyl substituent as directing group. Moreover the removal of the latter results in enhanced coplanarity of the p-conjugated polymer.
Despite the currently rather limited scope of the polycondensation reaction and the non-ideal atom efficiency, the authors point out for the first time an approach to suppress formation of branched and cross-linked structures during the direct arylation polycondensation via molecular design of the aromatic monomer. This protocol, therefore, paves the way for further studies including the investigation of various aromatic coupling partners and their optical and electrical characterization.
This article is part of the special Best of Macros 2013 issue, and is now free to read at http://www.best-of-macros.de!