The recent fervor over CO2 capture and conversion technologies, inspired by the effort to control greenhouse gas (GHG) emissions has often excluded methane (CH4), another major GHG contributor with a 100-year potency 30 times that of CO2. In particular, vented, leaked, and flared “fugitive” CH4 emissions from shale-gas wells are shaping up to be a major contributor to climate change. The big question is, what are we going to do with the rapidly increasing concentrations of CH4 and associated combustion product CO2 building up in our atmosphere?
The recent fervor over CO2 capture and conversion technologies, inspired by the effort to control greenhouse gas (GHG) emissions has often excluded methane (CH4), another major GHG contributor with a 100-year potency 30 times that of CO2. In particular, vented, leaked, and flared “fugitive” CH4 emissions from shale-gas wells are shaping up to be a major contributor to climate change. The big question is, what are we going to do with the rapidly increasing concentrations of CH4 and associated combustion product CO2 building up in our atmosphere?
The Other Greenhouse Gas
A grand challenge for projects aimed at mitigating the two most potent greenhouse gas emissions, CH4 and CO2, is to use renewable forms of energy to simultaneously convert them, using a small number of catalytic steps, into value-added chemical energy carriers. Ideally, this process could be executed in a single stage. Success in this endeavor would provide an effective way of transforming renewable electrical, solar, or thermal energy into stored chemical energy, which can be used for load-levelling on the electrical grid or as a synthetic fuel replacement for fossil fuels.
With this strategy of “killing two birds with one stone”, the two most potent anthropogenic greenhouse gases, CH4 and CO2, which are rapidly accumulating in our atmosphere, can be simultaneously reduced, while creating a usable chemical feedstock, such as CO/H2 synthesis gas. This synthon, known as syngas, can be transformed with known catalysts and processes, to methanol CH3OH and dimethyl ether (CH3)2O, for applications as a feedstock for making a myriad of chemicals and a clean-burning replacement for polluting diesel fuel.
Using this approach, CH4 flaring, venting and leakage, a common occurrence at the natural gas well head, can be eliminated and fossil generated CO2 can be reduced and valorized, both contributing positively to the reduction of greenhouse gas emissions in the war against climate change.
To expand upon this vision, in the light of climate change and the world’s growing energy demands, it has become imperative to reduce global CO2 emissions and seek alternatives that could ultimately end our dependence on fossil fuels. This year the concentration of CO2 in our atmosphere reached the unprecedented level of 404 ppm, compared with the 297 ppm pre-industrial revolution levels. To meet the Paris Cop 21 global warming targets this rate of increase in CO2 emissions must be curtailed by 2030.
In this spirit, research groups around the world have been actively pursuing the discovery and evaluation of novel catalytic nanomaterials capable of producing sustainable chemicals and fuels, from the plethoric quantities of anthropogenic gaseous CO2 present in our atmosphere. They have already been quite successful in the discovery of novel , scalable and inexpensive metal oxide based nanomaterials, which enable the thermochemical, photochemical or photothermal conversion of CO2 to CO, CH4 and CH3OH with high conversion rates, selectivity’s and turnover frequencies. Industry is in the throes of figuring out where CO2 utilization fits into its supply chain and how best to turn CO2 into a profitable chemical and fuel business that can compete with fossil resources.
The CO2 Scylla is Here, Now Enter Charybdis
As well as the CO2 challenge, we are also facing a dangerous anthropogenic CH4 problem, with atmospheric concentrations having reached around 2000 ppb, compared to pre-industrial revolution levels of about 700 ppb, with no sign of abatement. Note that, pound-for-pound, CH4 traps about 85 times the amount of solar heat than CO2, meaning its global warming potential is 85. Taking into account that CH4 in the atmosphere is gradually destroyed by solar radiation compared to CO2 slowly dissolving into the oceans and absorbing into soil and rock, the 100 year global warming potential of CH4 is estimated at about 28-36 times that of CO2.
All of this means the global warming equivalent of anthropogenic CH4 in our atmosphere, compared to CO2 is now roughly 60 000 ppb = 60 ppm and rising. At the current rate of increase, the effect of anthropogenic CH4 on our climate, exacerbated by the recent boom in shale-gas production and exploration, will soon catch up and surpass today’s unprecedented CO2 concentrations of 404 ppm. So we have to face the reality of a growing CH4 greenhouse gas climate change challenge as well as the CO2 challenge.
To amplify, about 20% of the CH4 concentration in our atmosphere stems from the production of coal, oil and natural gas, another 50% comes from other anthropogenic sources, such as fermentation, cultivation, biomass burning, animal waste, sewage treatment, and landfills, while the remainder emanates from natural sources that include permafrost, ocean and termites.
Therefore about 30% of CH4 comes from natural sources, which may be difficult to control, particularly if permafrost melts further releasing huge quantities trapped therein.
Every year, around 150 billion cubic meters of natural gas equivalent to 5.3 trillion cubic feet, is wastefully flared at thousands of oil fields globally. The total burned is equivalent to around one quarter of the annual gas consumption of the US. This results in more than 300 million tons of CO2 being emitted to the atmosphere, equivalent to emissions from approximately 77 million cars. If this amount of gas were used for power generation, it could provide more electricity (750 Billion kW-hr) than the annual consumption of Africa. Currently, natural gas is flared for a variety of technical, regulatory, and economic reasons because capture is not given high priority.
In the US, fueled largely by technological advances and the associated boom in shale-gas extraction, the production of natural gas has increased by more than 20 percent in the last five years. Currently there exist around half a million natural gas wells and thousands of miles of pipelines, with no sign of the shale-gas explosion slowing down. While there is some uncertainty regarding the exact amount of fugitive CH4 emitted from a natural gas well, it has been estimated that in CO2 equivalents over a one hundred year timeline, the CH4 GHG effect in the US will supersede the sum total of GHG emissions from all US iron, steel, aluminum and cement manufacturing facilities combined.
North American governments are clamping down on the practice of flaring, but oil producers are often left without economic options for dealing with this gas. The “Zero Routine Flaring by 2030” initiative endorsed by 9 countries, 10 oil companies and 6 development institutions was launched in April 2015 by UN Secretary-General Ban Ki Moon and World Bank President Jim Yong Kim. The endorsers collectively represent more than 40 percent of global gas flaring, with Canada and the United States within the top 10 grouping.
In oil-rich regions across the globe, many of which are located in remote areas of the country, surely it makes sense to catalytically convert fugitive CH4, directly at the well-head, into a storable and transportable liquid energy carrier, such as methanol, rather than continuing the current practice of venting and flaring the CH4 with its associated greenhouse gas problems.
The Fight Begins With the Catalyst
Dry reforming of methane with CO2 (CO2 and CH4 to CO and H2) has been undertaken using noble metals (Rh, Pd, Pt, Ru) for years. Commercially, firms have used nickel (Ni) based catalysts, but have created problems with coking via the Boudouard reaction. Without the appropriate catalyst, the dry reforming reaction requires temperatures above 1475 K. Recent work on “Super Dry-Reforming” uses a nickel based catalyst while supplying oxygen using a sacrificial iron oxide (Fe2O3) combined with MgAl2O4 operating at 1023 K. Since many sources of fugitive methane come from decentralized sources (1000-10 000 Nm3/day), any conversion process will require even lower temperature catalysts operating at near atmospheric pressures, creating a low parasitic load, while possibly using renewable energy (solar, wind, run-of-the river hydro, geothermal, etc.) to run and energize a dry-reforming system that yields an easy to store and a low-cost liquid fuel intermediary.
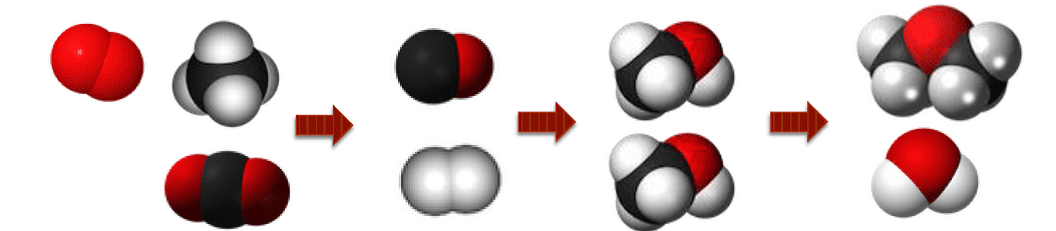
Illustration of the chemically catalyzed conversions, left to right, of CH4/H2/CO2 to CO/H2 to CH3OH to (CH3)2O.
There have already been some notable successes in the discovery of earth-abundant and inexpensive nanomaterials, which enable the gas-phase heterogeneous catalytic conversion of CO2 to CO and CH4 with high conversion rates, selectivity and turnover frequencies, energized by sources of heat and/or light. Today the materials chemistry and chemical engineering community are faced with the task of developing energy efficient and economically viable gas-phase heterogeneous catalysis processes, using renewable forms of energy, able to convert CH4, CO2 and CO into readily storable, transportable and usable value-added chemicals and fuels.
If such an advance can be transformed into a scalable, commercially viable and globally significant technology, the result would be the reduction in both CH4 and CO2 anthropogenic greenhouse gas emissions in “one fell swoop”. At the same time this breakthrough would create a usable chemical feedstock to make methanol CH3OH and dimethyl ether (CH3)2O, both able to be used as value-added chemicals and fuels, the latter being favored these days by the trucking and power generator industries as a clean combustion replacement in compression ignition engines, eliminating highly polluting petroleum diesel.
Currently, Chinese and European automotive companies have developed methanol (M15, M85 and M100) automobiles that effectively utilize wood alcohol mixtures in spark-ignition engines. Unlike electric cars using lithium based batteries, vehicles using methanol and dimethyl ether can be fuelled in few minutes, last for 10’s of years and can be almost completely recycled. More importantly, methanol and dimethyl ether vehicles can be built for under $15 000 in North America, making clean vehicles available for most citizens. Today’s electric car is a “nice to have” for wealthy buyers in industrialized nations with extensive grids. The global transportation emissions problem requires a low cost global solutions that uses locally produced, easy to store, renewable fuels for a vehicle that can be recycled easily.
Enter the Methanol Economy
There are convincing arguments why (CH3)2O is regarded as a clean energy source for the next generation of fuels. It can be handled like propane fuel; it generates fewer exhaust pollutants such as NOx, hydrocarbons, carbon monoxide and soot, the latter as there are no C-C bonds; its global warming potential is considerably lower than diesel; and it has the highest well-to-wheel efficiencies after natural gas. A challenge for the catalyst materials and chemical process engineering community is to discover a way to directly convert syngas CO/H2 into (CH3)2O in a single step process rather than having to proceed through two-steps involving the production followed by the dehydration of CH3OH.
Ideally, a low temperature, low pressure photo-catalytic process chain powered by renewable energy could be developed to convert CH4 and CO2 to CH3OH and/or (CH3)2O. Ideally, this systems’ parasitic load would be powered by solar power. Since a photo-catalytic process would operate in a semi-continuous mode, the separation columns could also operate semi-continuously, yielding reduced energy consumption during the separation and purification stages.
Methanol is a global chemical sold into a global market, 66 million tonnes being produced yearly with a current value of over $21 billion. The methanol market is supported by a global logistic chain stretching from Cape Horn to Inner Mongolia to the Baltic Sea. Converting methanol to (CH3)2O is an easy process. Moreover, since (CH3)2O is handled like propane, (CH3)2O can also leverage the global propane infrastructure. In the methanol economy, there is no need for cryogenic storage and high pressure carbon fibre tanks. Moreover, unlike CNG and LNG, there is no change for CH4 venting within the logistic chain.
Global (CH3)2O capacity is currently about 10 million tonnes. China Energy Limited is the largest producer of dimethyl ether in the world, delivering nearly 880 000 tonnes per year. The company uses liquid phase dehydration technology to produce DME and sells (CH3)2O to LPG distributors who blend it into propane, and the result is a product with improved combustion. China Energy also produces CH3OH. Current non-Asian (CH3)2O producers include Oberon, Mitsubishi, Shell, Grippo, and Akzo Nobel. Non-Asian producers typical use a two-step process, dehydrating methanol after methanol synthesis.
Living With the New Natural Gas
Although oil prices are in decline, global diesel and natural gas prices are diverging. Natural gas prices will continue to remain low due to horizontal drilling and hydraulic fracturing. On an energy equivalent basis, diesel and heating oil prices (10 $/GJ) will likely remain significantly higher than shale produced natural gas prices (3$/GJ) in the future.
Canada is the world’s fifth-largest natural gas producer and has enough natural gas reserves to meet current national energy demand for 300 years. The continuing price spread between refined oil products and natural gas will provide an ongoing competitive advantage for power sources using natural gas as feedstock. Currently, coal generation is being phased out and replaced by natural gas and some renewable sources for baseload operation. Under the shadow of Fukishima and a self-induced moratorium on North America hydro, natural gas is the baseload power leader.
Canadian federal and provincial governments will continue penalizing carbon dioxide emissions. With abundant, low cost natural gas feedstock, Canada can deploy thermally integrated poly-generation units, producing power and fuels, while capturing CO2 and supported by renewable energy sources. Facilities that reduce and recycle CO2 emissions, using additional renewable energy, will emerge as an important component of governmental energy policy and will receive preferential treatment from national and local authorities.
Low natural gas prices have supported a weak North American economy for the last decade, supplying inexpensive power and heat to households and industry.
It seems to me that if the increasing level of anthropogenic CH4 being released into our atmosphere, exacerbated by the shale-gas boom, is neglected, we could win the battle against CO2 emissions but end up losing the war on climate change!!!