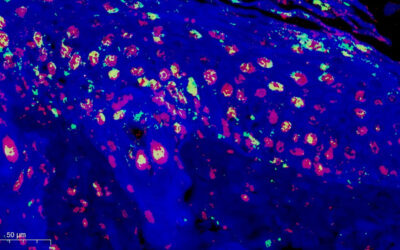
Since their invention roughly 400 years ago, microscopes using glass lenses and light have become irreplaceable in labs around the world. More powerful devices, like electron microscopes, do exist but cost millions and require expensive training and upkeep, making them unrealistic for most laboratories.
Labs therefore rely mostly on confocal microscopes, which use light and lenses to magnify objects. But the image loses focus when the size of the object being viewed approaches the size of the wavelength of light being used to observe it. At these scales, the object bends the light through a phenomenon called diffraction and distorts the image.
Microorganisms, such as bacteria, fungi, and viruses, exist in this size range and diffraction becomes a problem for microbiologists and pathologists attempting to research and diagnose them.
In the last eight years, a technique called expansion microscopy has improved the limitations of light microscopy, improving the resolution of conventional microscopes by, as the name suggests, expanding the microbes being viewed.
A group of biotech researchers lead by Yongxin Zhao at Carnegie Mellon University are now pushing its limits further.
Expanding microbes
Expansion microscopy expands a sample using what is called a polyelectrolyte hydrogel. These gels are networks of repeating molecules that are super absorbent and swell when water is added. Specific biomolecules like proteins in the sample are anchored to the gel matrix. As the gel swells, samples are likewise expanded up to four-fold, eliminating diffraction.
The challenge with expansion microscopy is ensuring uniform expansion of the sample, something that is hindered by the presence of a cell wall in many microbes — bacteria and fungi all have this rigid outer layer that prevents expansion. Furthermore, the biomolecules that are anchored to the gel each require a specific anchoring step, making the process more labor intensive and limiting the types of samples that can be expanded.
In June 2023, Yongzin Zhao published a new expansion microscopy platform consisting of a hydrogel that universally binds many biomolecules across the gel matrix. This provides a more uniform expansion of the samples and increased the types of organisms and tissues that could be viewed. However, two problems remained: the cell wall and their ability to label and identify specific structures.
The secret sauce for cell wall expansion
To solve the first problem, many have tried using enzymes to loosen or disrupt the cell wall, which alone had limited success. According to Zhao, his student Zhangyu Cheng, lead author of a new paper in Advanced Science describing the latest improvements to their expansion microscopy protocol named µMagnify, found optimal conditions to loosen the walls of a wide range of organisms.
“The secret sauce,” Zhao explained, “is a combination of heat treatment plus a cocktail of cell wall digesting enzymes.” With this recipe, they could expand a variety of pathogens and infected tissues including incredibly tough and problematic bacterial and fungal biofilms eight-fold.
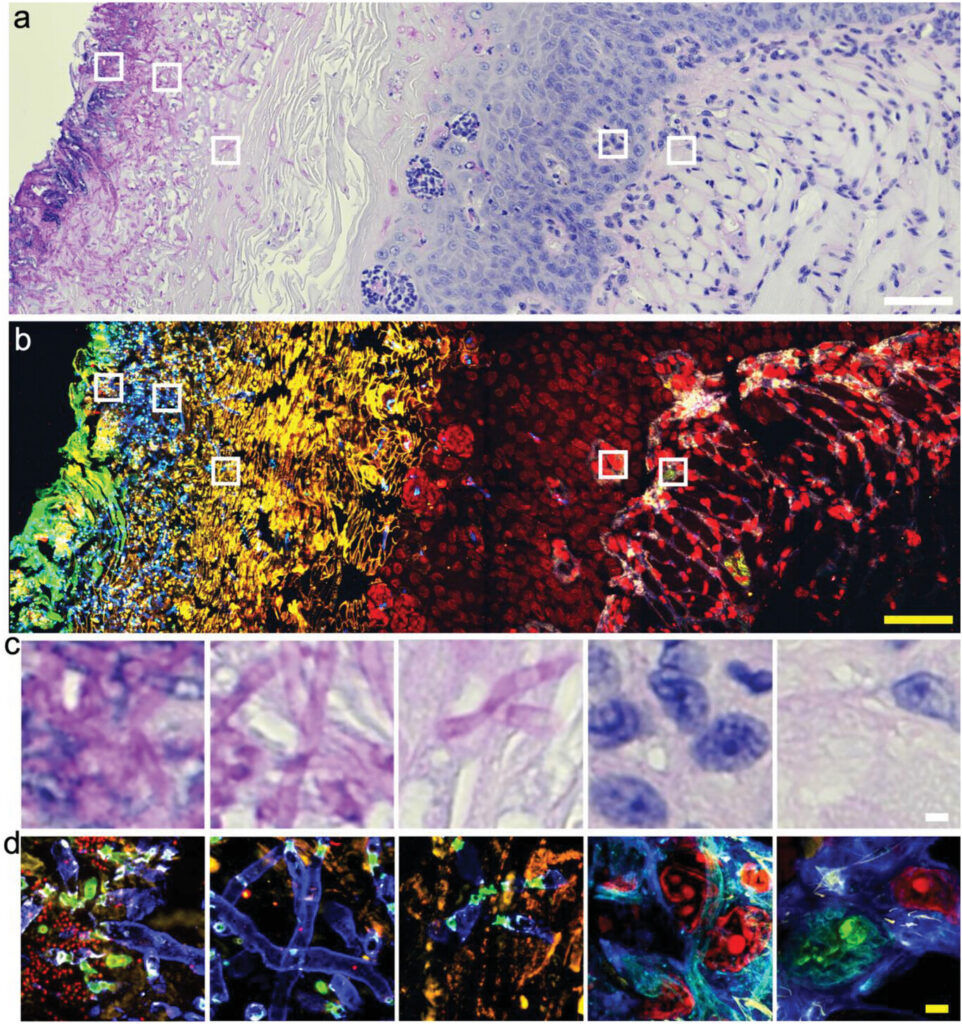
During active infections, many bacteria and fungi group together and produce an extremely dense protective layer called a biofilm. These defenses are difficult to penetrate and are one of the causes of drug resistant infections.
The µMagnify protocol improved the resolution of biofilms under the microscope giving researchers studying some of the most difficult infections for humans a big advantage.
Labeling proteins
The second challenge for microbiologists and pathologists is identifying key structures or proteins in the cells they’re observing. This allows them to identify specific microorganisms based on their unique proteins or demonstrate how a pathogen is interacting with host cells and causing illness.
For example, certain Escherichia coli bacteria produce a toxin that destroys intestinal cells, fluorescent labeling allows researchers to see these types of molecules and identify how and where they operate.
To do so, fluorescent labels are designed to target specific biomolecules and emit colored light once they bind. Labeling of a sample is usually constrained to four colors because of the wavelengths of the colored light begin to overlap meaning the colors blend together and are indistinguishable to the human eye.
“However, in our case, after you image these four colors, you can eliminate those labels and replace them with another four labels,” Zhao said.
This rinse-and-repeat ability allows for multiple rounds of labeling on a single sample. Couple this with the greater image expansion, and researchers can visualize multiple protein interactions and structures in 3D in a single organism or tissue section.
Cost effective and simple
The µMagnify system immediately increased the power and versatility of any confocal microscope already present in a lab. And according to Zhao, the cost is roughly $10 US dollars a sample and the protocol is so easy that, “any lab in the world can do it.”
For labs in places with fewer resources but many microbial pathogens, such as Sub-Saharan Africa or Southeast Asia, this system looks to be incredibly beneficial.
Zhao and his team even developed an immersive virtual reality program called ExMicroVR to visualize the data and allow multiple researchers to see and work simultaneously on the same sample.
Reference: Yongxin Zhao, et al. MicroMagnify: A Multiplexed Expansion Microscopy Method for Pathogens and Infected Tissues, Advanced Science (2023), DOI: 10.1002/advs.202302249