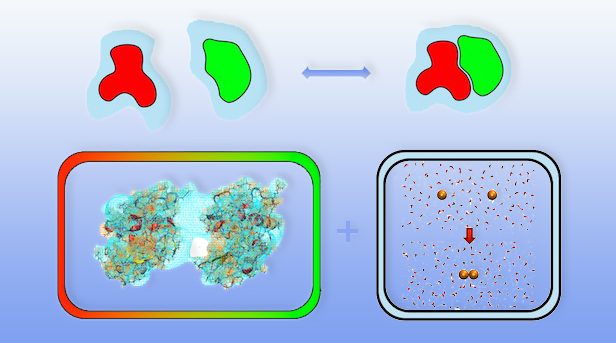
Solvation free energies contribute to the driving force of molecular processes in solution and play a significant role for the relative stability of biomolecular conformations or the formation of complexes. Changes in solvation free energy are the origin of solvent‐mediated interactions such as the hydrophobic effect in water.