Mesenchymal stem cells (MSCs) are used in hundreds of clinical trials for numerous indications, including bone and cartilage diseases, cardiovascular disease, and graft versus host disease (GVHD). Up to hundreds of millions of MSCs may be used per dose in ongoing trials, but fewer than 0.01% of cells in the bone marrow are MSCs. Therefore, there is a critical need to expand MSCs in sufficient numbers for treatment.
Several state-of-the-art biomanufacturing systems enable MSC expansion at high levels, and spheres of material called “microcarriers,” which provide support surfaces on which cells attach and grow, are among the most favorable. Microcarriers are currently used for growing MSCs in high numbers for scale-up applications. However, commercially available microcarriers are made from materials that are set by the manufacturer, such as polystyrene (a plastic), dextran (a polysaccharide), and gelatin (typically derived from animal collagen). These materials are not directly customizable for cell growth, nor to instruct other desirable cellular properties. Poly (ethylene glycol) (PEG) is an example of a hydrogel material that can be tailored to offer various levels of control over cellular attachment and growth properties.
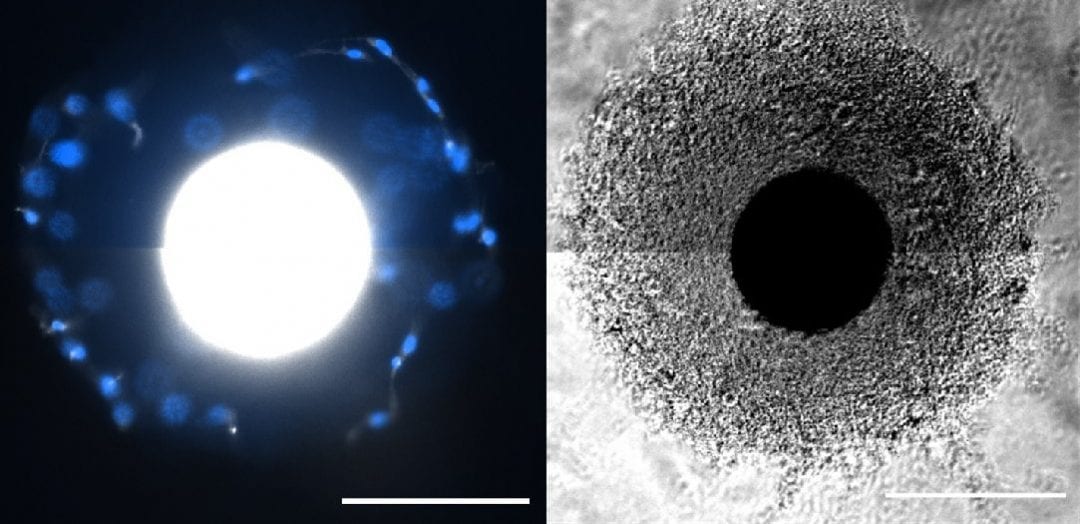
In Advanced Healthcare Materials, William L. Murphy and co-workers, University of Wisconsin-Madison, developed customizable PEG coatings on “core” microcarriers to support stem cell growth. The ability to customize sites to which cells can attach and hydrogel stiffness may be highly useful when designing new microcarrier systems. An “interfacial polymerization” approach coated microcarriers in a reproducible fashion, with initiation of the coating reaction at the “core” microcarrier surface. Coated microcarriers supported MSC attachment and growth, with high expansion capacity and low population doubling times. MSCs were removed from microcarriers using the digestive enzyme, trypsin, or the much gentler ethylenediaminetetraacetic acid (EDTA), and retained their capacity to differentiate into bone/fat lineages using both removal approaches in serum-free media. It is now possible to manufacture functional MSCs in chemically defined media in a scalable manner.