More than 2,000 gene therapy clinical trials have been completed since they started in 2015, and Glybera, a treatment for a rare inherited disorder, became the first gene therapy drug to be approved by the European Medicines Agency in 2012. Kymriah, a genetically modified immune cells from patients to attack their cancer, was approved as the first gene therapy treatment in the United States by the Food and Drug Administration (FDA) in 2017.
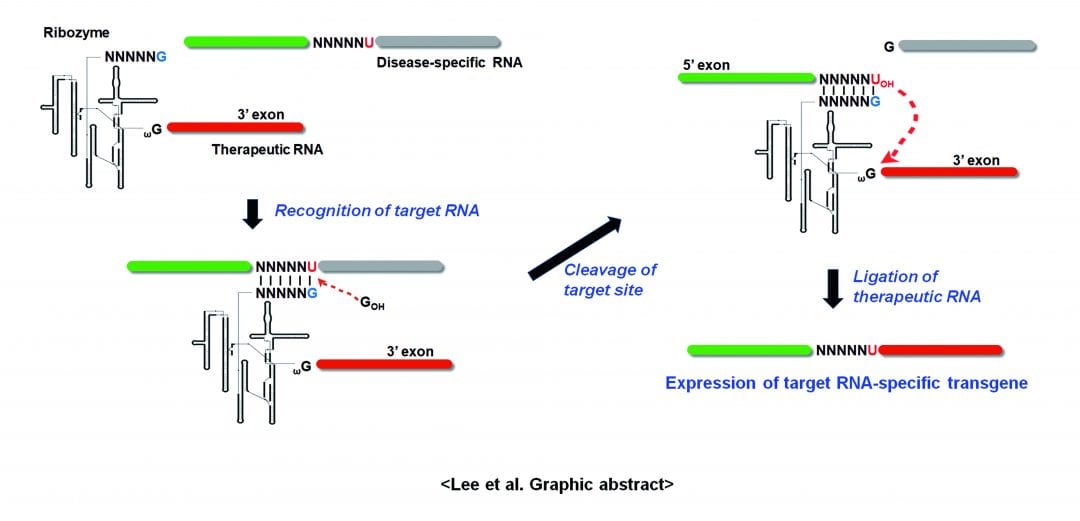
Although there have been some successes in gene therapeutic approaches, more specific, efficient, and safe tools are needed to successfully fight most of diseases caused by variation at the DNA, RNA, or protein level. In a recently published review article in WIREs RNA, “Therapeutic applications of group I intron-based trans-splicing ribozymes,” Lee et al. reviews and discusses the current status of the trans-splicing ribozyme as an emerging gene therapeutic approach with high efficacy and specificity. Trans-splicing ribozyme has been engineered and improved from naturally existing group I introns to edit and replace disease-causing RNAs into corresponding wild-type or entirely new genes for therapeutic purposes.
There are two types of applications using the trans-splicing ribozymes: repair and reprogramming. Ribozymes for repairing can replace a mutation in the substrate RNA with the non-mutated sequence, and those for reprogramming can replace target RNA with a therapeutic sequence or a reporter sequence to circumvent diseases or for imaginging-associated RNA expression. Using these properties, various disease-associated RNAs have been targeted and repaired and/or reprogrammed to treat genetic, infectious, and malignant diseases and therapeutic outcomes were verified using diverse cell culture and animal systems. This ribozyme will be more advantageous than many traditional gene therapeutic methods because it simultaneously allows the reduction of disease-associated gene expression while inducing therapeutic gene activity specifically in cells expressing the target RNA.
Recently, an adenovirus-mediated delivery and tissue-specific promoter system combined miRNA regulation has been shown to yield the selective and efficient expression of trans-splicing ribozymes in target tumor cells and tissues, thus facilitating the reprogramming of target RNAs and exerting the desired therapeutic effects in various cancer models. Consequently, a clinical cancer trial has recently begun in Korea to test the hTERT-targeting trans-splicing ribozyme as a cancer gene therapeutic against hepatic metastasis of solid tumor. Although trans-splicing ribozyme-based therapeutic applications may require additional refinement, continued optimization or modification processes will hopefully soon result in improved trans-splicing ribozymes with diverse applications in various clinical conditions.
Kindly contributed by the Authors.