Polymer-based materials are used in a large range of applications from light-weight structural composites and coatings to functional materials in very diverse sector s like transportation, medical, and electronics. However, the use of polymers can be limited as they sometimes suffer, for instance, from low damage tolerance and impact strength. In addition, they usually exhibit high melt viscosities which render them difficult to be processed. These drawbacks are partly related to the intrinsic structure of polymeric materials constituted of high molecular weight covalent chains with long relaxation times.
s like transportation, medical, and electronics. However, the use of polymers can be limited as they sometimes suffer, for instance, from low damage tolerance and impact strength. In addition, they usually exhibit high melt viscosities which render them difficult to be processed. These drawbacks are partly related to the intrinsic structure of polymeric materials constituted of high molecular weight covalent chains with long relaxation times.
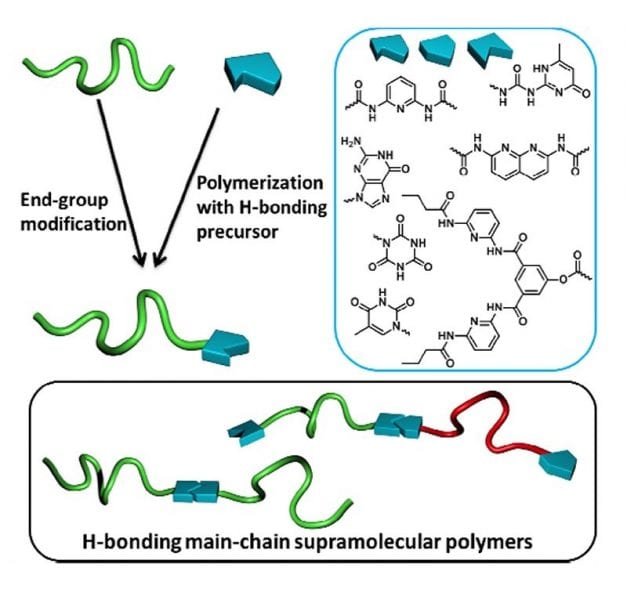
One way of overcoming these difficulties may be to involve supramolecular concepts in macromolecular science. While traditional chemistry is based on covalent bonds, supramolecular chemistry relies on non-covalent interactions (hydrogen-bonds, π-π or ionic interactions, metal coordination…) to design spontaneously-assembled edifices. This research area is inspired by Nature, DNA being the most striking example of supramolecular assembly with a double helix stabilized by hydrogen-bonds between nucleotides. Among physical interactions, hydrogen bonds have attracted interest due to both their high sensitivity to external stimuli (temperature) and to the wide range of binding units that were developed in recent decades. Due to the dynamic character of physical bonds, supramolecular systems exhibit valuable properties like stimuli-responsiveness or self-repair which are not observed in covalent counterparts. Polymer materials may benefit from these properties if supramolecular binding units are introduced at the extremities or along macromolecular chains.
A recent review by Arthur Bertrand and co-workers reports on current advances in synthetic strategies intending to end-functionalize polymer chains by hydrogen-bonding supramolecular stickers. Combining the reversibility of non-covalent interactions with the intrinsic macroscopic properties of covalent polymers opens new perspectives for the design of polymer materials with a wealth of interesting properties.