Phosphorylase kinase (PhK) is a complex, multimeric serine/threonine-specific protein kinase that stands out among other kinases for its role of stimulating glycogen catabolism and releasing free glucose-1-phosphate by phosphorylating and activating glycogen phosphorylase, a fundamental enzyme in glycogen metabolism. In fact, PhK is the only enzyme known to perform this function.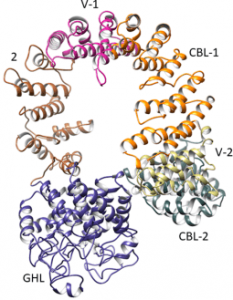
In the human body, the cells of the liver and skeletal muscle are largely responsible for synthesis and storage of glycogen. Therefore, PhK defects and deficiencies can lead to a glycogen storage disease with mild to severe metabolic myopathies, exercise intolerance, enlarged liver or heart, low blood sugar, and many others.
Recent work suggesting an involvement of phosphorylase kinase in cancer and memory consolidation has sparked a renewed interest in its structure and regulation. PhK is a large complex having sixteen subunits, (αβγδ)4, with the activity of its catalytic γ subunit being controlled allosterically by its α, β and δ regulatory subunits.
No high-resolution structure of the intact complex, nor of its regulatory α and β subunits, has been solved. Of its four types of subunits, the least is known about the 138.4 kDa α.
In their Protein Science paper, Owen Nadeau, Gerald Carlson and their colleagues from the University of Kansas Medical Center and the University of Michigan have combined an integrative approach of modeling and hydrogen-deuterium exchange of the intact complex to gain structural information on PhK’s a subunit. These results show it to be a multi-domain helical molecule with several possible intersubunit contact sites, including with the catalytic γ subunit.
This news article was written by Owen Nadeau and Gerald Carlson.
The featured image courtesy of Mary Ashley Rimmer.