Most commercial polymers are synthesized via radical polymerization. For the design of the polymer structure and of the appropriate polymerization process knowledge of polymerization kinetics is indispensable.
For decades, it has been the common opinion that the kinetics of radical polymerization is more or less unaffected by the solvent nature – contrary to ionic polymerization – as long as the reaction is chemically and not diffusion controlled. This view changed dramatically with the emergence of the PLP-SEC (pulsed-laser polymerization – size exclusion chromatography) technique which allows for a precise experimental determination of the propagation rate coefficient in radical polymerization. The wide-spread application of this technique to radical polymerization in various solvents revealed; however, that there actually exist very significant solvent effects in radical polymerization. Depending on the system – solvent(s), monomer and their concentrations – the measured apparent propagation rate coefficient may increase, but also decrease, by a factor of 10 or even more when switching from bulk monomer to rather dilute concentrations or between different solvents. Even though well-proven experimentally, and despite various attempts to explain this effect at least qualitatively, the origin was not fully understood from a theoretical viewpoint, and no quantitative prediction was possible.
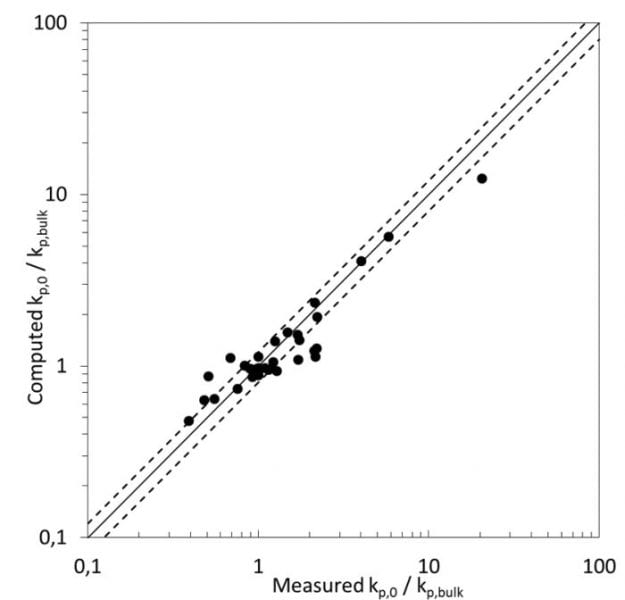
Recently, K.-D. Hungenberg (from the University of Paderborn) together with P. Deglmann and H. Vale (from BASF SE) reported a novel view to this effect. They applied classical Eyring transition state theory to the propagation step in radical polymerization, and successfully correlated the ratio of computed activity coefficients γ of all species involved – (γMγP*)/γTS (M = monomer, P* = propagating radical and TS = transition state) – with the reduced propagation rate coefficient kp/kpB. The prediction of activity coefficients of these (partially transient) species was performed here with the quantum chemistry based solvation model COSMO-RS.
As a consequence, the solvent effect can be entirely rationalized by the degree of thermodynamic non-ideality of the system, and this explanation also covers the behavior for complex or less common solvents like water or ionic liquids, and some unusual monomers like iso-bornyl methacrylate. In addition to offering a sound explanation of the solvent effect, this new approach (experimental determination of the rate coefficient in bulk combined with COSMO-RS or similar methods) drastically reduces the effort to explore the solvent effect on kp. Typically, it takes less than a day to compute the change of activity coefficients with medium composition, whereas the respective PLP-SEC experiments might require several weeks or months for even a skilled experimentalist.