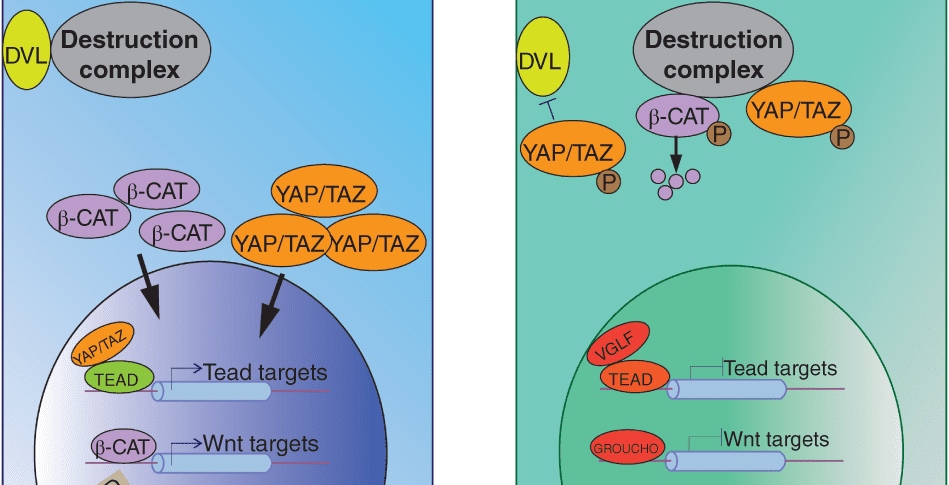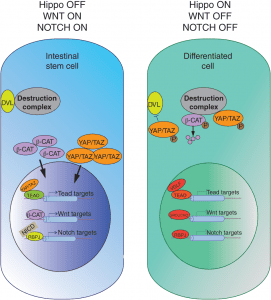
Hippo signaling interactions. More here.
The intestine is a vital organ for food absorption. The average length of the human intestine is approximately five meters, and the cell surface area of the intestine is estimated to be the size of half a badminton court. Intestinal cells covering this surface are renewed every 3–5 days by the activity of stem cells located in pocket-like gland structures called crypts. These stem cells are capable of differentiating into all essential intestinal cell types to maintain the healthy organ and restore it when damage occurs. The improper function of these stem cells can lead to a myriad of diseases, such as cancer and inflammation. Constant exposure to a toxic environment puts them at a high risk of injury. Remarkably, when intestinal stem cells are damaged, other differentiated cells in the crypt quickly become able to revert to stem cells, displaying extraordinary cell fate flexibility. This recent review in WIRES Developmental Biology, by Smith et al., discusses the intrinsic and extrinsic mechanisms underlying intestinal cell plasticity.
The microenvironment of intestinal stem cells, otherwise known as the stem cell niche, provides signals to support the growth and differentiation of stem cells. Recent studies have revealed that mesenchymal cells surrounding stem cells provide contextual information, in the form of key cell signaling cascades, to modulate stem cell activity. While these extrinsic signals establish and reinforce stem cell differentiation, differentiated cells retain the intrinsic ability to revert to stem cells when necessary. Recent work has shown that this is accomplished through various modifications of DNA accessibility that permit changes in gene expression. This review summarizes how these forces—signals, environment, and DNA accessibility—govern the function of intestinal crypt cells, and identifies the challenges the field faces in applying these findings to human disease.
Contributed by Tae-Hee Kim.