 Analysis of a composite catalyst system by researchers from the University of Toronto could have major repercussions for the development of solar fuel reactors. These devices, which use sunlight to convert CO2 into fuels such as hydrogen and methane, are at the forefront of a scientific movement to turn CO2 emission from a potential environmental disaster to a useful chemical feedstock. Their work was published in the new Wiley journal Advanced Science.
Analysis of a composite catalyst system by researchers from the University of Toronto could have major repercussions for the development of solar fuel reactors. These devices, which use sunlight to convert CO2 into fuels such as hydrogen and methane, are at the forefront of a scientific movement to turn CO2 emission from a potential environmental disaster to a useful chemical feedstock. Their work was published in the new Wiley journal Advanced Science.
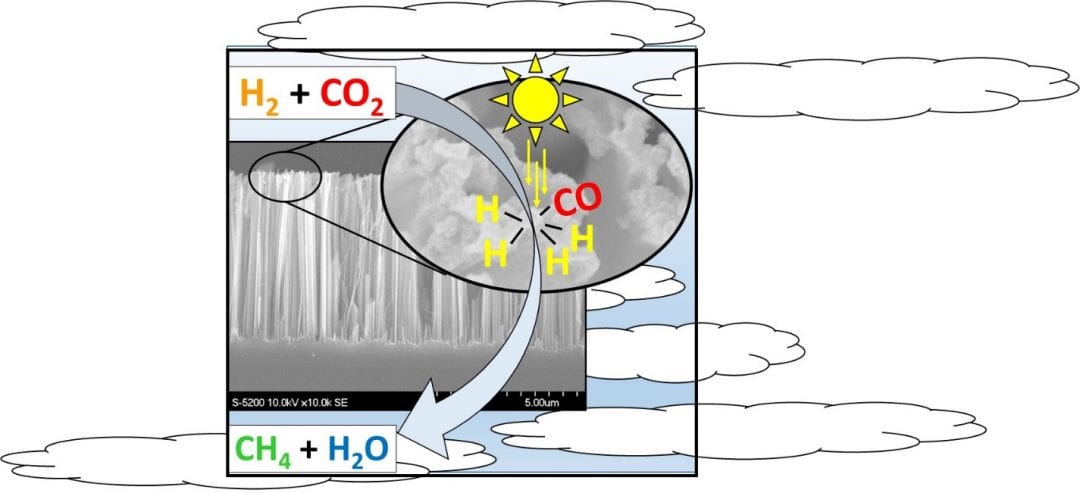
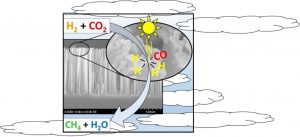
Professor Geoffrey Ozin and his team of researchers showed that ruthenium nanoparticles sputtered onto black silicon nanowire supports activate CO2 conversion in a hydrogen atmosphere under solar-simulated light. The group unraveled the effects of irradiation and heat on the catalytic activation,, making an important step towards engineering tandem solar reactors.
These reactors, which can be used to convert waste CO2 into useful fuels such as hydrogen or (as in this case) methane, are expected to play a major role in future sustainable energy generation. As well as reducing CO2 emissions at the source, they can be used to normalise energy supplies from inherently variable power sources, such as wind or solar, and could even be utilised as part of a system to remove excess CO2 from the atmosphere.
The Toronto group found that their ruthenium/nanowire catalyst heats up upon irradiation with solar-simulated light, and this increased temperature allowed for increased reaction rates. From a photochemical standpoint, at a fixed temperature the reaction rate increases proportionally to the number of incident photons with an energy greater than the band gap of silicon. The group come to the conclusion that photogenerated charge carriers in the silicon support might facilitate the formation of active hydrogen participating in the reduction of CO2. This means the photochemical contribution to the overall reaction rate is significant, even if only a small portion of photogenerated charge carriers contributes to the photomethanation reaction.
The benefit of using silicon nanowires as support for the catalysis lies in their broad absorption in a wide range of the solar spectrum, while minimal reflection occurs when they are vertically etched into silicon wafers (hence their name, “black silicon”). Ruthenium, on the other hand, is a relatively expensive chemical – the group suggest that further work should be committed to replacing it in this system with something less expensive, such as nickel. This, they believe, would assist greatly in the development of large-scale reactors, an essential technology for reducing CO2 emissions in the future.