 Delving into biology, even at a superficial level, is an amazing experience for gaining profound respect for the intricacies of homeostasis. Every discovery at the bio-molecular level brings a wave of inter-related variables that each impart their influence; their role in maintaining our health. It boggles the mind trying to understand the wonderfully natural, systematic functions that tick along un-noticed (and often all too unappreciated), let alone to believe it can all work with such precision.
Delving into biology, even at a superficial level, is an amazing experience for gaining profound respect for the intricacies of homeostasis. Every discovery at the bio-molecular level brings a wave of inter-related variables that each impart their influence; their role in maintaining our health. It boggles the mind trying to understand the wonderfully natural, systematic functions that tick along un-noticed (and often all too unappreciated), let alone to believe it can all work with such precision.
At the organism level, these millions of processes are working in harmony, but imagine the view at the molecular scale of the workings of a cell. Thousands of molecular species bustling about their business. The molecular scale, without the greater viewpoint, looks to be in a manner of contemporary chaos. Nature is a master of chaos, however; with chaos theory defining that processes in an apparently disordered state are in fact operating with an underlying order and purpose that cannot be discerned by conventional observation. The appearance of disorder can alternatively be taken as being analogous to a measure of our lack of understanding.
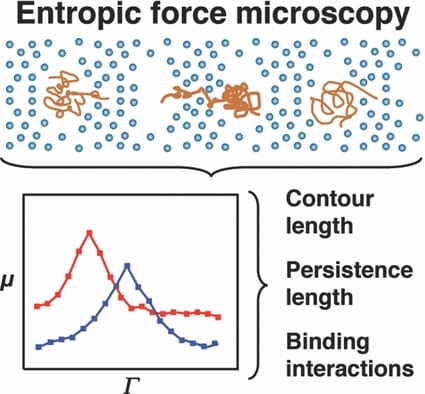
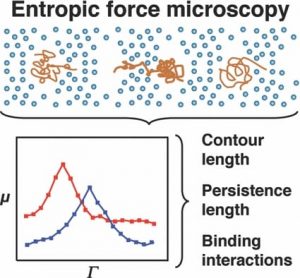
Researchers at the Texas A&M University are now improving our understanding of DNA interactions with small molecules as a function of system entropy (which can be taken statistically as a measure of disorder). By confining individual molecules, they have observed dynamics that could otherwise be discounted as noise. However, careful observation reveals the influence of molecular conformation and rigidity on molecular movement.
A molecule entering a confined space finds itself with fewer conformational options to be able to fit in that space. This reduces the conformational freedom and hence the apparent possible ways we may perceive it to be disordered. A cost of energy is needed for this to occur. In the work of Shi and Ugaz, this is imparted to the DNA fragments via an electric field. The consequent ease of transport is monitored as the DNA molecule is forced to squeeze through nano-sized pores. Changes in the molecule size and rigidity by binding with other molecules changes the nature of the force needed to assist in the DNA’s transport through the pores.
With this improved observation, comes improved understanding and an increasing appreciation of the ‘chaos’ being a well tuned, highly-dependant system, that can assist in explaining the most fundamental processes in biology from the molecule to organ level. Gene expression, critical in this balance, is dictated by protein and enzyme binding to DNA. How a molecule uniquely interacts and recognises a specific DNA sequence is instrumental in maintaining the underlying homeostatic order.
Cancer and other morbidities however eventuate with a loss of this delicate balance. Here, drugs and other molecules can interfere with these interactions and potentially supress genetic disorders, or stimulate the bodies own defences. Similarly, understanding how drugs, such as chemotherapies, interact with DNA can elucidate how they actually function. These interactions also play roles in biomedical engineering and diagnostics fields.
Entropic force microscopy is set to significantly improve our understanding of orderly biomolecule interactions for healthy function.