The technology chosen to provide the energy for powering electric vehicles (EV) is the lithium battery. Electrochemists and solid chemists, who are at the heart of the scientific community interested in lithium batteries, have always sought to find new active materials with increased intrinsic performance. Another important aspect was the study of the compatibility of these active materials with the liquid electrolyte. A much less explored research path is the optimization of electrode formulation and fabrication.
The active materials cannot function alone as electrodes, except in microbatteries where they are then used in thin layers of low capacity. In general, the active materials are used as micrometric grain powders and formulated with various additives such as an electronically conductive agent of nanometric size (traditionally a carbon black powder) and a polymeric binder (glue). Electrode films are thus composite materials that must be studied as a whole in order to optimize them.
The energy density of the batteries is limited because the volume occupied by the inactive components (current collectors, separator, conductive additive, binder, and electrolyte) is too large. This is because the electrodes are limited in thickness for kinetic reasons. Indeed, at the scale of the active material grains, the oxidation-reduction reactions can be ultra-fast. However, there are significant limitations to the charge transport kinetics (ions and electrons) through the electrode. For a long time these transport properties remained poorly understood due to the lack of tools to characterize them. Thus, the formulation of the electrodes has been optimized using a trial-error and intuition approach. Numerical simulations have also been developed, but they are based on simplified electrochemical models from the point of view of electrochemical reactions as well as electrode geometry.
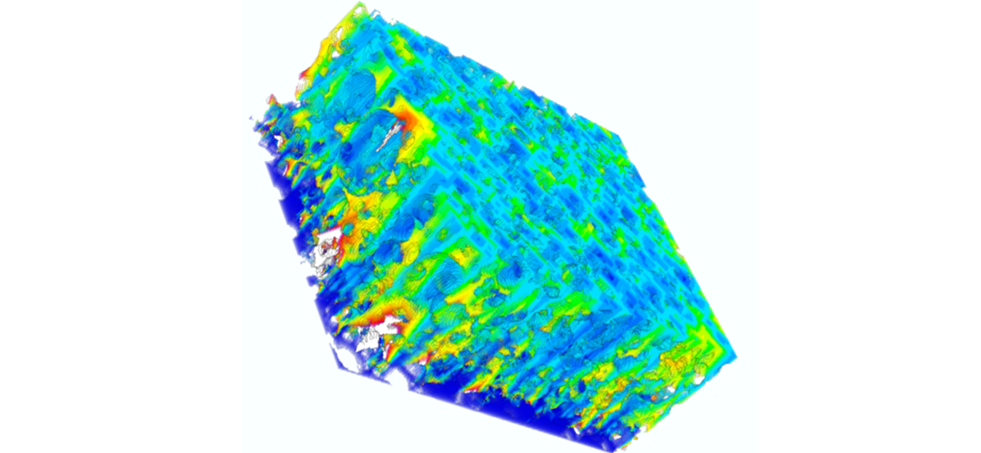
Recently, the development of tomographic techniques made it possible to quantify the 3D geometry of the electrodes. But no work has connected the parameters describing the microstructure of the electrodes with both measurements of their transport properties and their electrochemical performance. In this article, Bernard Lestriez and co-workers used X-ray tomography and focused ion beam in combination with scanning electron microscopy (FIB-SEM) tomography for a multiscale analysis of the 3D geometry of electrodes of a design suitable for EV. The electronic transport properties were measured by broadband dielectric spectroscopy (BDS), which is an ultra-powerful technique because it allows a description of electrical properties at different scales (grain, cluster, electrode film). Electrochemical performance was measured at different rates of discharge and at different temperatures.
This approach facilitates the reliable identification of various causes which limit the kinetics of the electrodes. At moderate discharge regimes, typically greater than 1 hour, the quality of electronic transport governs performance. At discharge regimes of less than 1 hour, and for electrodes in which the electronic transport is good, performance is limited by the transport of the lithium ions into the electrolyte. The key parameters of the electrode geometry with respect to the transport properties were identified and target values quantitatively determined.
This work opens the way to a more rational optimization of the composition and the 3D geometry of the electrodes for maximizing their performance and the energy and power densities of the lithium batteries.