Techniques to generate asymmetric nanoparticles present a greater challenge than standard bottom-up colloidal particle syntheses. The particle surface must be engineered to have at least two distinct chemistries with a defined boundary.
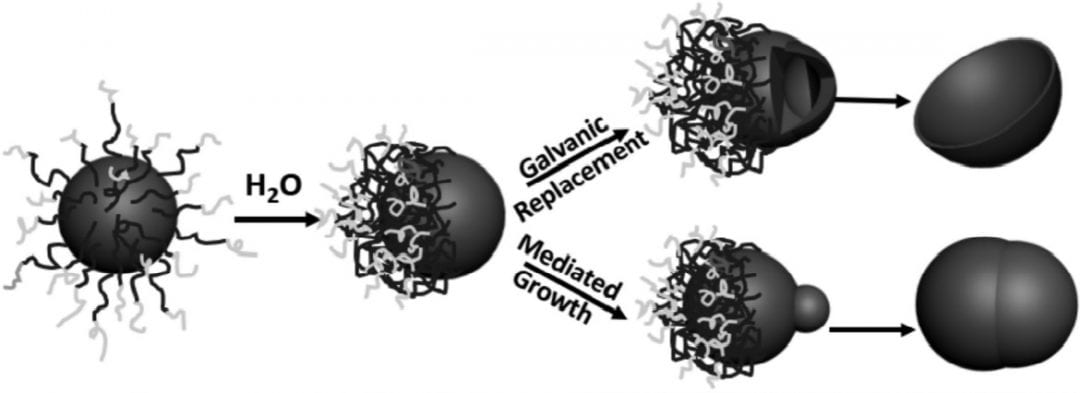
A method developed at the University of Connecticut, USA, involves the manipulation of polymer tethers on the surface of gold and silver nanoparticles to generate asymmetric metal nanoparticles directly in an aqueous solution. In their paper in Small, they describe two techniques: one to create metal nanobowls, and one to create Janus-type metal dimers.
The first step in both reactions is to attach the block co-polymer poly(ethylene oxide)-block-polystyrene tethers to the particles and adjust their position on the surface by adding water to the solution. The polystyrene is very hydrophobic, so the addition of water causes the tethers to bunch up on the particle surface, leaving one side of the spherical particle free.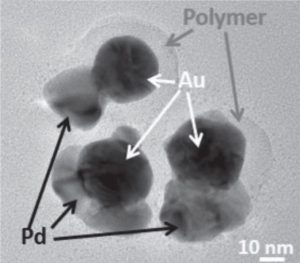
This unprotected side of the particle can then undergo further reaction. These researchers demonstrate the galvanic replacement of half-protected sacrificial silver spheres to form gold nanobowls, and they use half-protected gold particles as seeds to form more complex metal structures with palladium. The mechanisms for the formation of both types of particles are thoroughly investigated and explained in their communication article, available online now.