Three-dimensional (3D) bioprinting is a technique that seeks to fabricate functional artificial tissues and organs that biologically resemble their counterparts in vivo. The technology is crucial to heal or replace damaged or necrotic tissues/organs, potentially fulfilling organ needs for transplants, and provide new platforms for drug testing and studying tissue morphogenesis.
In this technology, 3D structures composed of hydrogels simulating the extracellular matrix are fabricated based on layer-by-layer deposition of biological materials, biochemicals and living cells. One of the challenges in 3D bioprinting is to reach the high resolution needed for hollow tubular structures, since the internal diameters can be very small (e.g., 8-20 µm in blood vessel capillaries and venules).
Four-dimensional (4D) bioprinting is a recently developed technology based on 3D bioprinting; however, it also integrates stimuli-responsive biomaterials, fabricating active constructs that can alter their shapes upon stimulation to achieve prescribed functionality. Previously, shape-changing polymers were deposited first, followed by cells. Moreover, the use of mostly hydrogels made from thermoresponsive (meth)acrylates, did not meet the need for biocompatible or biodegradable.
Professor Ionov and colleagues recently reported in Advanced Materials an advanced 4D biofabrication approach based on simultaneous printing of polymer–cell bioinks to form shape-morphing biopolymer hydrogels. The approach allows for the fabrication of hollow self-folding tubes with control over their diameters and architectures at high resolution.
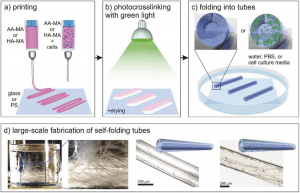
Scheme of the 4D biofabrication of self-folding hydrogel-based tubes. a) Printing of polymer solutions. b) Crosslinking of the printed films with green light. c) Instant folding upon immersion in water, PBS, or cell culture media. d) Schematic illustrations and representative microscope images of single tubes with/without printed cells; photograph of a glass vial containing a large number of self-folded tubes (for large-scale production). More here.
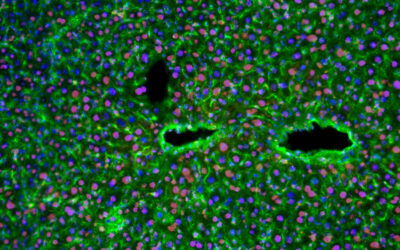
As a proof of concept, methacrylated alginate (AA-MA) and hyaluronic acid (HA-MA) hydrogels with suspended mouse bone marrow stromal cells were used. The polymers are sensitive to Ca2+ ions and are crosslinked with a gradient crosslinking density, which allows for reversible shape transformations. Internal tube diameters as low as 20 µm were achieved, which is not yet possible with other approaches. Moreover, the process does not pose any negative effect on the viability of the printed cells, with cell survival for at least 7 days without any decrease in cell viability.
The authors hope their approach can be used to create tailored, cell-laden, shape-morphing architectures for tissue engineering and regenerative medicine applications.