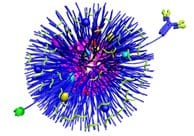
By copying some of the body’s own delivery systems, it may be easier to combat a whole range of diseases. Researchers at the Wooley Laboratory have had some new success in using polymers to mimic the characteristics and actions of certain biological nanoparticles and structures, such as proteins, lipoproteins, and viruses.
These synthetic recreations not only travel the blood and body in the same way as their biological counterparts, but also gain access to cells in the same way, potentially allowing a whole new range of diagnostic and treatment modalities with vast implications. In addition to allowing the delivery of nucleic acids, drugs, genes, and chemotherapies, the nanostructures can also help diagnose disease by delivering dyes and imaging probes.
One of the main challenges in development of these therapeutic nanoparticles has been the premature release of the enclosed drug, due to breakdown of the particle itself. The new research finds a way around this by making the particles more stable through a technique called shell- and core-crosslinking—building up a small particle into a larger polymerized object and slowing down the release of any enclosed drugs. In fact, drugs and other therapeutics delivered via these polymers have been observed being released over a timeframe as long as several days, while minimizing damage to healthy tissues.
a way around this by making the particles more stable through a technique called shell- and core-crosslinking—building up a small particle into a larger polymerized object and slowing down the release of any enclosed drugs. In fact, drugs and other therapeutics delivered via these polymers have been observed being released over a timeframe as long as several days, while minimizing damage to healthy tissues.
Increases in polymer chemistry are allowing for an incredibly diversity of applications. An ever greater control over the functioning of these synthetic particles, even more so than over their natural counterparts, helps to avoid the disadvantages that normally come with them, such as insertional mutagenesis, limited cargo size, and difficulties in storage and scalability. Additional features can also be built into the polymers, allowing them to respond to environmental or physiological triggers, such as releasing drugs at certain temperatures or at the acidic pH of a tumor site.
Different regions of the structures can also be “programmed” with different functions, allowing a variety of interactions, and the delivery of more than one type of cargo. For instance, the structures can carry and deliver multiple therapeutic or diagnostic tools at one time, and can use more than one targeting mechanism to deliver them. Targeting mechanisms include either active targeting with specific cell recognition, or more passive targeting through sites with leaky vasculature, such as tumor tissues. By loading together imaging probes and drugs in the same particle, it is possible to provide diagnosis and treatment at the same time.
The two main advantages to these synthetic nanoparticles appear to be that they are more flexible and can be designed for several applications, and they less immunogenic and can be injected several times, unlike their natural counterparts. However, these synthetic nanoparticles still have limitations, namely that their efficiency lags behind that of the natural particles.