Bladder cancer is most commonly diagnosed in patients who experience hematuria (blood in urine). Patients with hematuria are evaluated for bladder cancer using invasive tests with low sensitivity and specificity, even though only less than 30% of them are eventually diagnosed with cancer. Therefore, there is a pressing need for a low-cost non-invasive test that can quickly evaluate these patients for bladder cancer.
Researchers at the University of Pittsburgh led by Steven R. Little have addressed this need by designing a clinical assay that detects increased proteolytic enzyme (matrix metalloproteinase, MMP) activity in the urine of bladder cancer patients. In their recent publication in Advanced Healthcare Materials, they describe this assay, called “Ammps”, as a switchable system which turns ON in the presence of active collagenase in the urine. It is based on gelatin-crosslinked-alginate nanoparticles hiding the Fe(II) catalyst, which becomes available when the gelatin is degraded by collagenase activity in the urine of bladder cancer patients. This leads to the resuspension of Fe(II)-carrying alginate nanoparticles in a proportional manner. These resuspended alginate nanoparticles are then treated with acid, thereby releasing the catalyst which can oxidize a chromophore dye generating a visual signal.
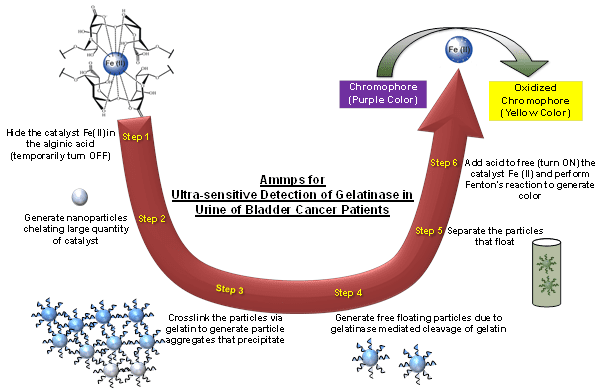
Strategy behind Ammps for the detection of increased proteolytic activity in the urine of bladder cancer patients.
The group already used Ammps in a pilot trial for the diagnosis of bladder cancer in patients referred for hematuria evaluation. This study revealed that Ammps had 100% sensitivity, 85% specificity and a negative predictive value of 100% for bladder cancer diagnosis. Given its lower cost and higher sensitivity compared to current point-of-care tests, Ammps could be the diagnostic test of choice in the future, after confirming these results in a larger trial.

















