Light from the sun on earth, while the most abundant form of energy, suffers from its low energy density (about 1 kW/m2 of land) and therefore cannot at present, replace fossil fuels. Moreover, a large fraction of the solar spectrum on earth is composed of infrared and visible light (weak energy) further limiting its application. Focusing on the photocatalytic hydrogen production from water, as an energy carrier, several factors need to be considered. These include materials stability, energy requirement both in magnitude (band energy) and alignment (band edge) in addition to kinetic considerations. Over the last two decades, many approaches were undertaken to solve these requirements. The review focus is on work related to changing the sun-light spectrum from low to high energy and therefore making it possible to use sable wide band-gap semiconductor materials for energy conversion.

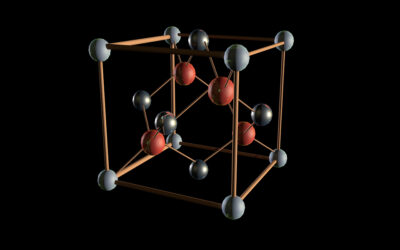
A: a schematic representation of a plasmon-upconverter-catalyst triple junction material for converting water to hydrogen and oxygen using sunlight. Water is adsorbed on the surface of a wide band gap semiconductor catalyst and can be split to hydrogen and oxygen molecules photo-catalytically using the small fraction of UV and near UV light from the sun. The large fraction of light in the visible and IR penetrates the catalytic material interact with the plasmonic material and is thus amplified. This amplified light is then converted to high energy photons that can back excite the semiconductor making more hydrogen and oxygen. B: the absorption and emission spectra of an up converting nanocrystals. These materials are generally comprised of an inert host material (often NaYF4) doped with sensitizer (such as ytterbium, Yb3+) and activator (Thuluim, Tm3+) lanthanide ions. Yb3+ ions are excited by a 980 nm light is conveniently resonant with the f−f transitions of Tm3+, facilitating efficient energy transfer leading to upconversion emission bands at 345, 365, 451, 481, 646, and 800 nm.
This can be done by the use of the so-called “up-conversion luminescence materials”. The uniqueness of these up-conversion materials is their potential to convert a specific weak energy range to a higher one. However, the process that has been initially observed by Auzel[i] in 1964 is inefficient. To increase its efficiency another process can be brought and that is of plasmonics. The field of plasmonics has seen tremendous work since the pioneering work of Al-Sayed[ii]. In short, plasmonic materials are substances that can enhance dramatically the electric field and therefore are poised to concentrate the effect of light at the vicinity of a given material. The challenge is of how to put these two materials (the plasmonic and up-conversion materials) then put them in contact with a wide band gap semiconductor catalyst to split water to hydrogen and oxygen using sun light. For practical applications, a rate of 10-6 and 10-5 g-moles of reactant per second per cm3 of volume of reactor as indicated by Weisz[iii] is the target.
A review article recently published by Khan and Idriss is a compilation of work conducted in this direction and shows that indeed some progress has been made by nano-design of hybrid materials. Yet, many questions, challenges and unknown factors are still pertaining. In particular, those related to interface plasmonic/up-converter, the tuning of the plasmonic to the emission or absorption light range of the up-converter and to the engineering of the three materials (plasmonic, up-converter and photo-catalyst) to make a single device for energy production as indicated in the figure below.
Kindly contributed by Hicham H. Idriss.
References:
[i] F. Auzel, Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 104, 139-174 (2004)
[ii] S. Link, M.A. El-Sayed, The Journal of Physical Chemistry B 103 (40), 8410-8426 (1999)
[iii] P.B. Weisz, The Science of possible, Chemtech, 424-425 (1999)